A system and method for preparing high-purity low-valent vanadium oxides by chlorination
A vanadium oxide and chlorination technology, applied in the fields of metallurgy and chemical industry, can solve the problems of discomfort, large environmental pollution, and greatly different operation methods and conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
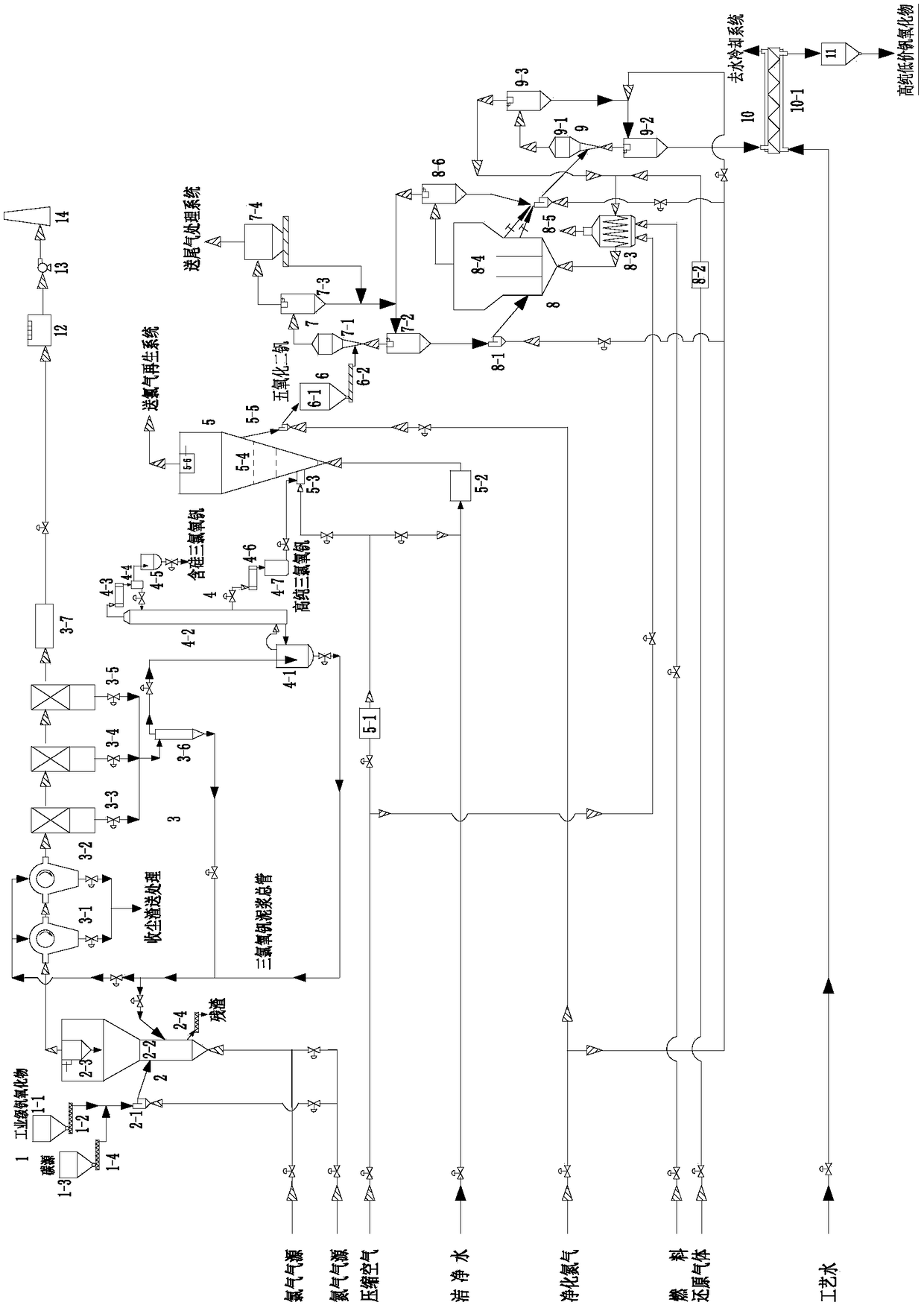
[0075] The chlorination method used in this embodiment prepares the system of high-purity low-valent vanadium oxide, such as figure 1 As shown, it includes feeding section 1, chlorination section 2, dedusting and rinsing section 3, rectification and purification section 4, catalytic oxidation section 5, catalytic oxidation product feeding section 6, preheating and dust removal section 7, control reduction section 8, first-level Cooling section 9, secondary cooling section 10, high-purity low-price vanadium oxide silo 11, tail gas leaching absorption tower 12, induced draft fan 13, chimney 14;
[0076] The feeding section 1 includes an industrial-grade vanadium oxide silo 1-1, an industrial-grade vanadium oxide screw feeder 1-2, a carbon source silo 1-3, and a carbon source screw feeder 1-4;
[0077] The chlorination section 2 includes a chlorination bed feeder 2-1, a chlorination fluidized bed main body 2-2, a chlorination bed cyclone separator 2-3 and a chlorination bed spira...
Embodiment 2
[0098] The method for preparing high-purity low-valent vanadium oxides using the system described in Example 1 comprises the following steps:
[0099]The industrial-grade vanadium oxide in the industrial-grade vanadium oxide silo 1-1 and the carbon source in the carbon source silo 1-3 pass through the industrial-grade vanadium oxide screw feeder 1-2 and the carbon source screw feeder 1-4 respectively. Enter the chlorination fluidized bed main body 2-2 after entering the chlorination bed feeder 2-1 mixing simultaneously; From the chlorine of the chlorine gas source main pipe, the nitrogen of the nitrogen gas source main pipe through the chlorination fluidized bed main body 2-2 bottom The air inlet enters the main body 2-2 of the chlorinated fluidized bed to maintain the fluidization of industrial-grade vanadium oxide and carbon source and chemically react with it. The chlorine gas and carbon source work together to produce industrial-grade vanadium oxide and a small amount of im...
Embodiment 3
[0106] In this example, powdered industrial-grade vanadium pentoxide (purity: 98.50%) is used as raw material, and the method described in Example 2 is used to prepare high-purity and low-valent vanadium oxide. Industrial grade vanadium pentoxide (purity 98.50%) has a processing capacity of 80kg / h, and is prepared by chlorination, dedusting and rinsing, rectification and purification of vanadium oxychloride, catalytic oxidation, and precise control of reduction to obtain high-purity vanadium trioxide products , with a purity of 99.995wt% (4N5).
[0107] In the main body 2-2 of the chlorination fluidized bed, the amount of petroleum coke added during the chlorination process is 30% of the mass of the industrial-grade vanadium pentoxide powder, the chlorination operating temperature is 600°C, and the operating gas velocity in the fluidization section is 3.0m / s, the mole fraction of chlorine in the chlorine-nitrogen mixed gas entering the air chamber is 20%; in the main body 5-4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com