A system and method for preparing highly active and high-purity specific valence state vanadium electrolyte
A high-purity, high-activity technology, applied in the fields of energy and chemical industry, can solve the problems of affecting the stability of vanadium ions, not directly applicable to the reactor, and large environmental pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
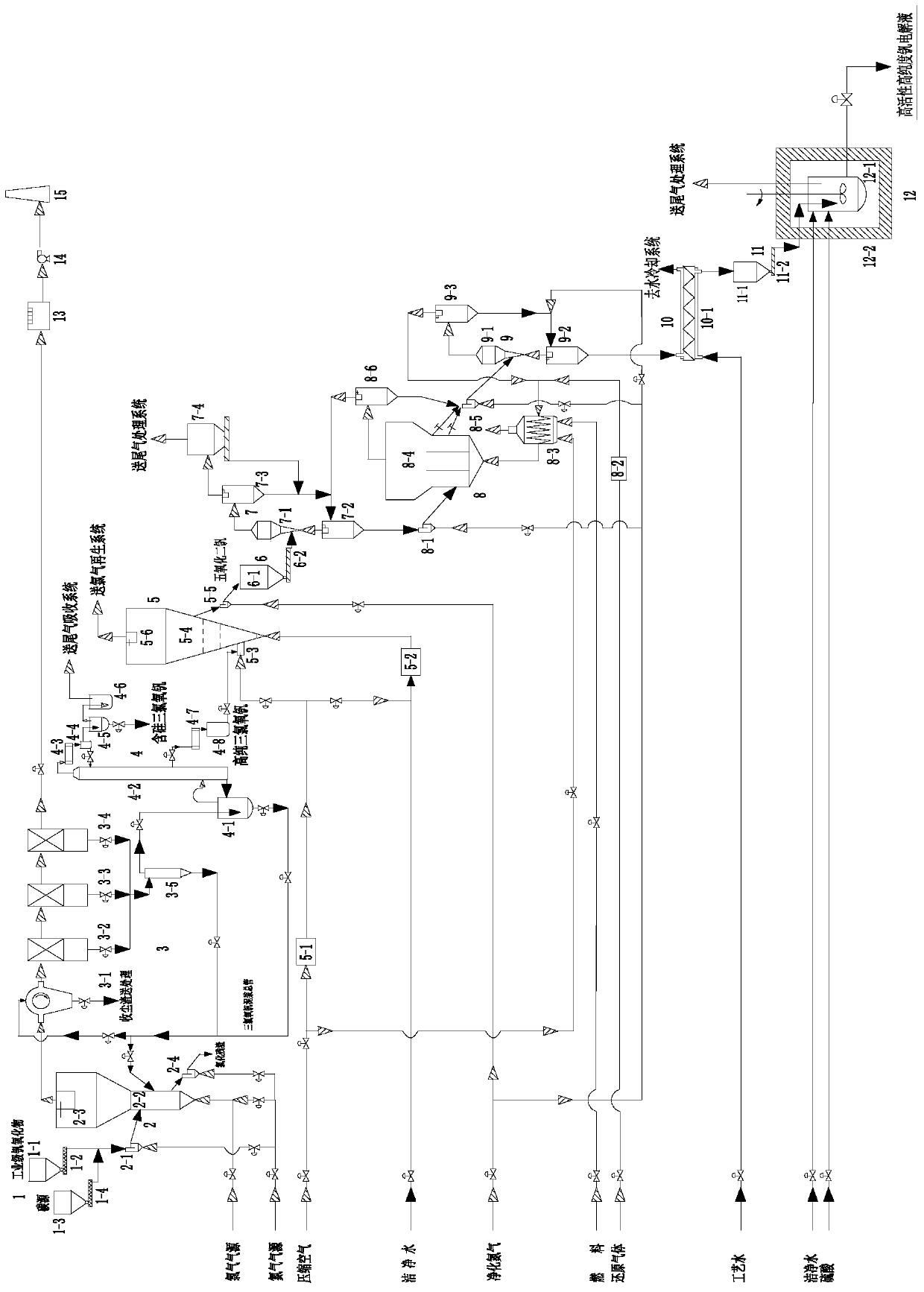
[0085] A system for preparing highly active and high-purity specific valence state vanadium electrolyte, such as figure 1 As shown, it includes feeding section 1, chlorination section 2, dedusting and rinsing section 3, rectification and purification section 4, catalytic oxidation section 5, catalytic oxidation product feeding section 6, preheating and dust removal section 7, control reduction section 8, first-level Cooling section 9, secondary cooling section 10, high-purity and low-price vanadium oxide feeding section 11, dissolution and activation section 12, tail gas leaching absorption tower 13, induced draft fan 14, chimney 15;
[0086] The feeding section 1 includes an industrial-grade vanadium oxide silo 1-1, an industrial-grade vanadium oxide screw feeder 1-2, a carbon source silo 1-3, and a carbon source screw feeder 1-4;
[0087] The chlorination section 2 includes a chlorination bed feeder 2-1, a chlorination fluidized bed main body 2-2, a chlorination bed cyclone ...
Embodiment 2
[0111] The method for preparing a highly active and high-purity specific valence state vanadium electrolyte using the system described in Example 1 comprises the following steps:
[0112] The industrial-grade vanadium oxide in the industrial-grade vanadium oxide silo 1-1 and the carbon source in the carbon source silo 1-3 pass through the industrial-grade vanadium oxide screw feeder 1-2 and the carbon source screw feeder 1-4 respectively. Enter the chlorination fluidized bed main body 2-2 after entering the chlorination bed feeder 2-1 together; From the chlorine gas of the chlorine gas source main pipe, the nitrogen of the nitrogen gas source main pipe through the lower part of the chlorination fluidized bed main body 2-2 The air inlet enters the main body 2-2 of the chlorinated fluidized bed to maintain the fluidization of industrial-grade vanadium oxide and carbon source and react with it, and the chlorine gas and carbon source work together to chlorinate vanadium oxide and a...
Embodiment 3
[0120] In this example, powdery industrial-grade vanadium pentoxide (purity: 98.50%) is used as raw material, and the method described in Example 2 is used to prepare a high-activity and high-purity vanadium electrolyte in a specific valence state. The processing capacity of industrial grade vanadium pentoxide (98.50% purity) is 80kg / h, and the equivalent The valence state is 3.0 high-purity and high-activity vanadium electrolyte.
[0121] In the main body 2-2 of the chlorination fluidized bed, the amount of petroleum coke added during the chlorination process is 30% of the mass of the industrial-grade vanadium pentoxide powder, the chlorination operating temperature is 600°C, and the operating gas velocity in the fluidization section is 3.0m / s, the mole fraction of chlorine in the chlorine-nitrogen mixed gas entering the air chamber is 20%; in the main body 5-4 of the catalytic oxidation fluidized bed, the water vapor fed into the catalytic oxidation process is 30% of the qu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com