Fluorescence probe for detecting bisulfite and preparation and application
A bisulfite and fluorescent probe technology, which is applied in the preparation of carboxylic acid nitriles, carboxylic acid esters, organic compounds, etc., can solve the problems of expensive instruments, complicated processing, and inability to monitor in real time, and achieve fast detection speed , good selectivity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
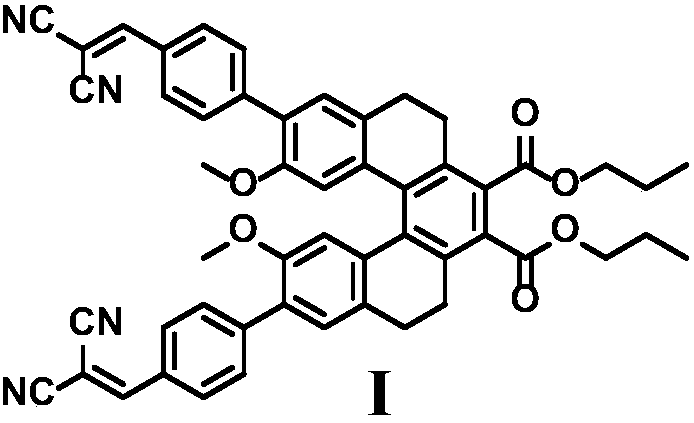
[0022] Embodiment 1: the preparation of molecular fluorescent probe I
[0023] (1) Compound 1 (76mg, 0.114mmol), add anhydrous potassium carbonate (0.157g, 1.14mmol), 4-formylphenylboronic acid (51.2mg, 0.342mmol), 15mL dimethylformamide, 10mL water, 13mg Tetrakistriphenylphosphine palladium was heated to reflux for 12 hours under the protection of nitrogen. Then it was extracted with ethyl acetate and purified by column chromatography to obtain compound 2 (70 mg, 85%).
[0024] (2) Add malononitrile (81.9 mg, 1.24 mmol) to compound 2 (0.3 g, 0.415 mmol), two drops of piperidine, and 20 mL of ethanol, and stir at room temperature for 2 hours under nitrogen protection. After purification by column chromatography, fluorescent probe I (0.25 g, 73%) was obtained.
[0025] Test results:
[0026] 1 H NMR (500MHz, CDCl 3 )δ7.95(d,J=8.4Hz,4H),7.78(s,2H),7.72(d,J=8.4Hz,4H),7.31(s,2H),6.77(s,2H),4.36– 4.21(m,4H),3.32(s,6H),3.14–3.06(m,2H),2.92–2.84(m,4H),2.76–2.66(m,2H),1.83–1.74(...
Embodiment 2
[0028] Example 2: Application of bisulfite ion fluorescent probe.
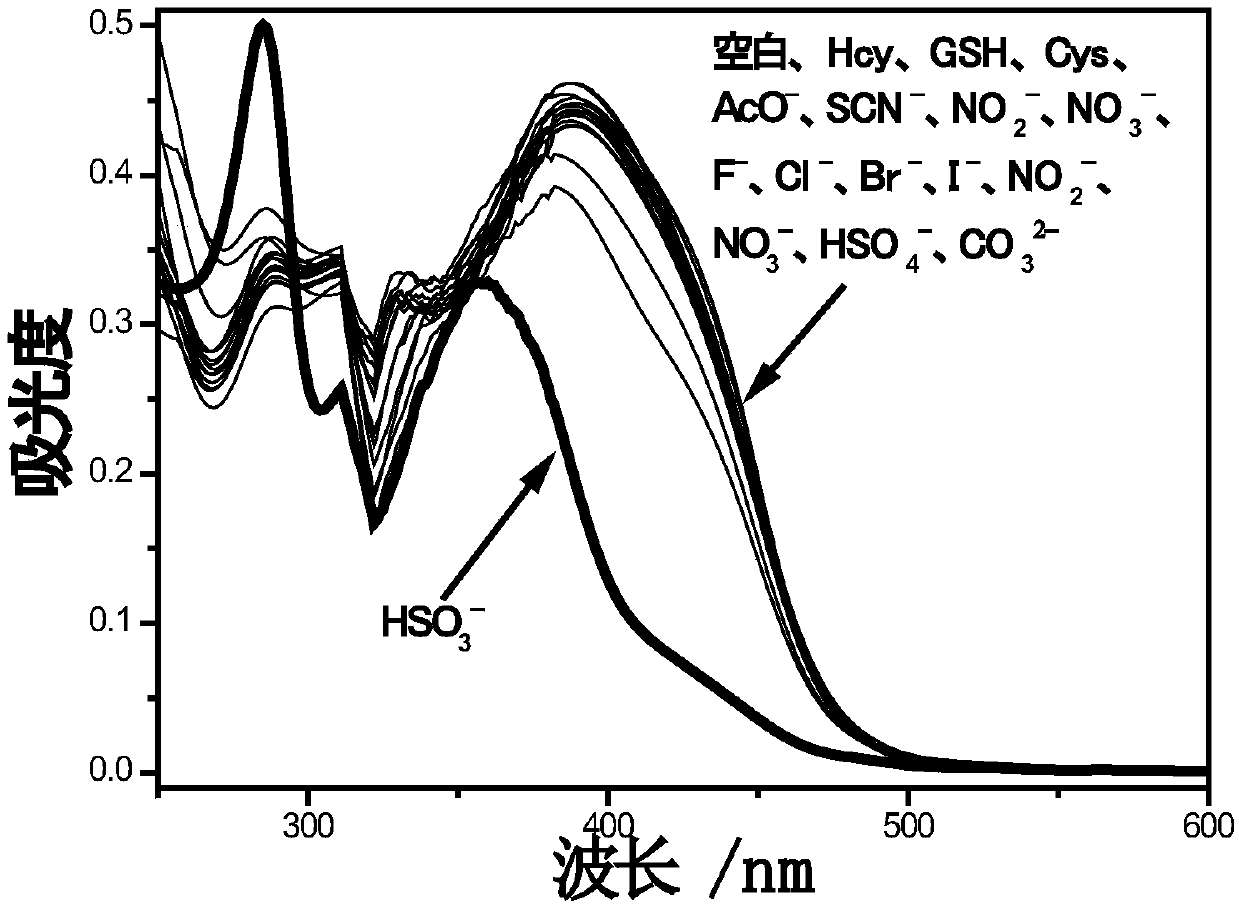
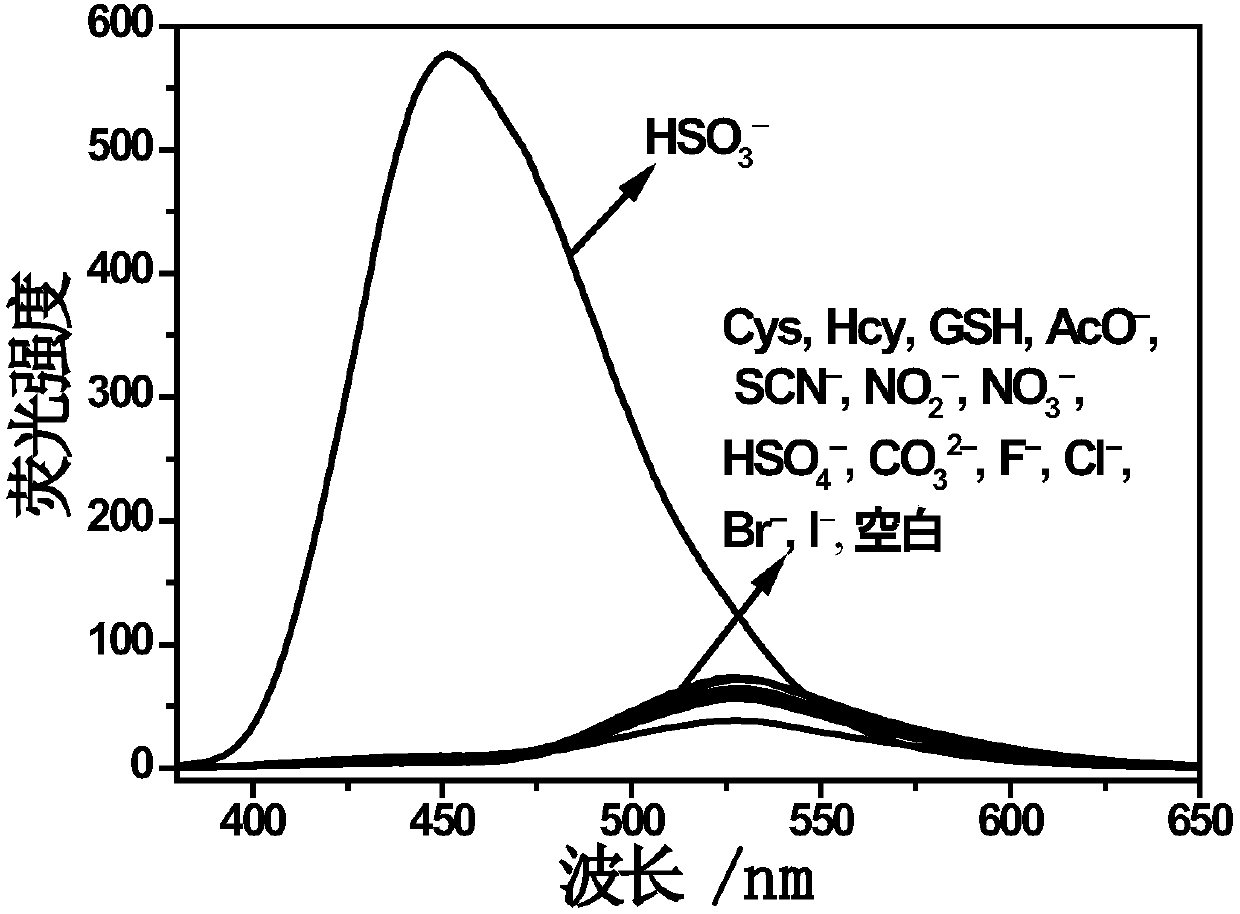
[0029] The Molecular Probe I was dissolved in dimethyl sulfoxide, configured as a probe solution of 1 mmol / L, and diluted into a mixed solution of ethanol and PBS buffer (dimethyl sulfoxide: PBS=1:1, v / v), using a UV-Vis spectrophotometer and a fluorescence spectrophotometer to probe I to identify HSO 3 - performance has been studied. The concentration of probe I was 1 × 10 -5 M and 5×10 -6 M, used for UV absorption and fluorescence tests respectively.
[0030] figure 1 It is the ultraviolet absorption spectrum after adding 40 equivalents of different anions and mercapto-containing compounds to the probe I solution. 1×10 -5 In the probe I solution of M, add 40 equivalents of different anions and mercapto compounds (acetate ion, thiocyanate ion, nitrite ion, nitrate ion, bisulfate ion, carbonate ion, fluoride ion, chlorine ion, etc. ions, bromide, iodide, cysteine, homocysteine, glutathione). It was f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com