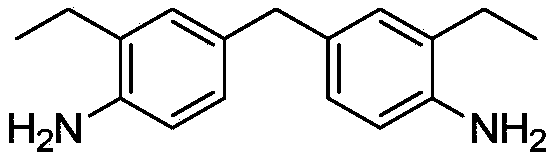
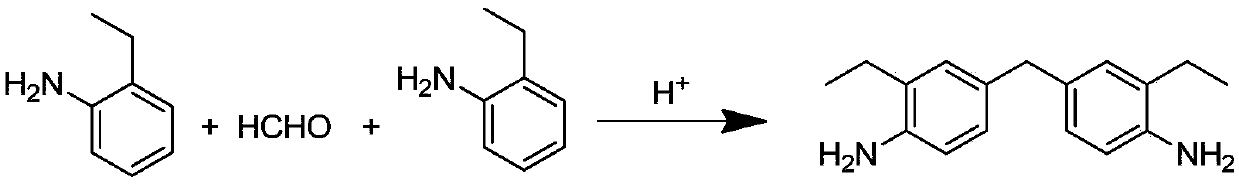
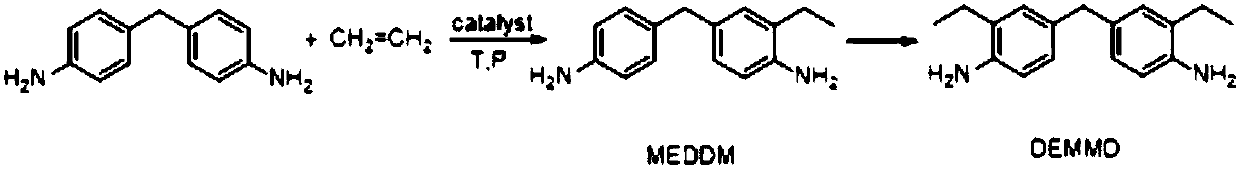
Catalyst for synthesizing DEMMA as well as preparation method and application thereof
A technology of catalyst and molecular sieve, which is applied in the direction of catalyst activation/preparation, molecular sieve catalyst, chemical instruments and methods, etc. It can solve the problems of strong acid corrosion, poor catalyst selectivity, low content of diaminodiphenylmethane, etc., and achieve the effect of inhibiting disproportionation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0080] Preparation of Catalyst A-1
[0081] (1) NaHCO 3 solution modification
[0082] Under airtight conditions, the HY molecular sieve was treated with NaHCO with a concentration of 0.2mol / L 3 The solution was stirred and reacted at 65° C. at a liquid / solid ratio of 40 ml / g. After 3 hours of reaction, the temperature was lowered to stop the reaction, filtered, and washed with deionized water until neutral to obtain the modified molecular sieve carrier M-1.
[0083] (2) Ce and B modification
[0084] Using the equal volume impregnation method, the modified molecular sieve carrier M-1 was impregnated in 100ml solution containing 2.549g cerium nitrate and 1.432g boric acid, impregnated for 12h to wait for adsorption equilibrium, then put it in an oven for 2h at 120°C, and finally transferred to Baking in a muffle furnace at 450°C for 6h. obtained with 0.1% CeO 2 and 0.8% B 2 o 3 Modified Carrier N-1.
[0085] (3) Using the equal-volume impregnation method, impregnate th...
Embodiment 1-2
[0087] Preparation of Catalyst A-2
[0088] (1) NaHCO 3 solution modification
[0089] Under airtight conditions, the mordenite molecular sieve was treated with NaHCO with a concentration of 0.1mol / L 3 The solution was stirred and reacted at 50°C at a liquid / solid ratio of 50ml / g. After reacting for 5 hours, the temperature was lowered to stop the reaction, filtered, and washed with deionized water until neutral to obtain the modified molecular sieve carrier M-2.
[0090] (2) Ce and B modification
[0091] Using the equal volume impregnation method, the modified molecular sieve carrier M-2 was impregnated in 100ml solution containing 1.794g cerium nitrate and 0.822g boric acid, impregnated for 10h to wait for adsorption equilibrium, then put it in an oven for 4h at 110°C, and finally transferred to Baking in a muffle furnace at 500°C for 8h. with 2.5% CeO 2 and 1%B 2 o 3 Modified Carrier N-2.
[0092] (3) Using the equal volume impregnation method, the modified molecul...
Embodiment 1-3
[0094] Preparation of Catalyst A-3
[0095] (1) NaHCO3 solution modification
[0096] Under airtight conditions, the Beta molecular sieve was stirred and reacted with NaHCO3 solution with a concentration of 0.4mol / L at a liquid / solid ratio of 60ml / g at 60°C. After 4 hours of reaction, the temperature was lowered to stop the reaction, filtered, and washed with deionized water until neutral. The modified molecular sieve carrier M-3 was obtained.
[0097] (2) Ce and B modification
[0098] Using the equal volume impregnation method, the modified molecular sieve carrier M-3 was impregnated in 100ml solution containing 2.575g cerium nitrate and 2.157g boric acid, impregnated for 16h to wait for adsorption equilibrium, then put it in an oven for 4h at 120°C, and finally transferred to Baking in a muffle furnace at 350°C for 8h. 2% CeO2 and 1.2% B 2 o 3 Modified Carrier N-3.
[0099] (3) Using the equal volume impregnation method, the modified molecular sieve carrier N-3 is imp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com