A kind of tumor actively targeting star-shaped amphiphilic polymer micelle nanomedicine and preparation method thereof
A technology of amphiphilic polymers and star polymers, which can be used in antineoplastic drugs, drug combinations, pharmaceutical formulations, etc., and can solve problems such as no obvious improvement in curative effect, no curative effect, and reduced curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
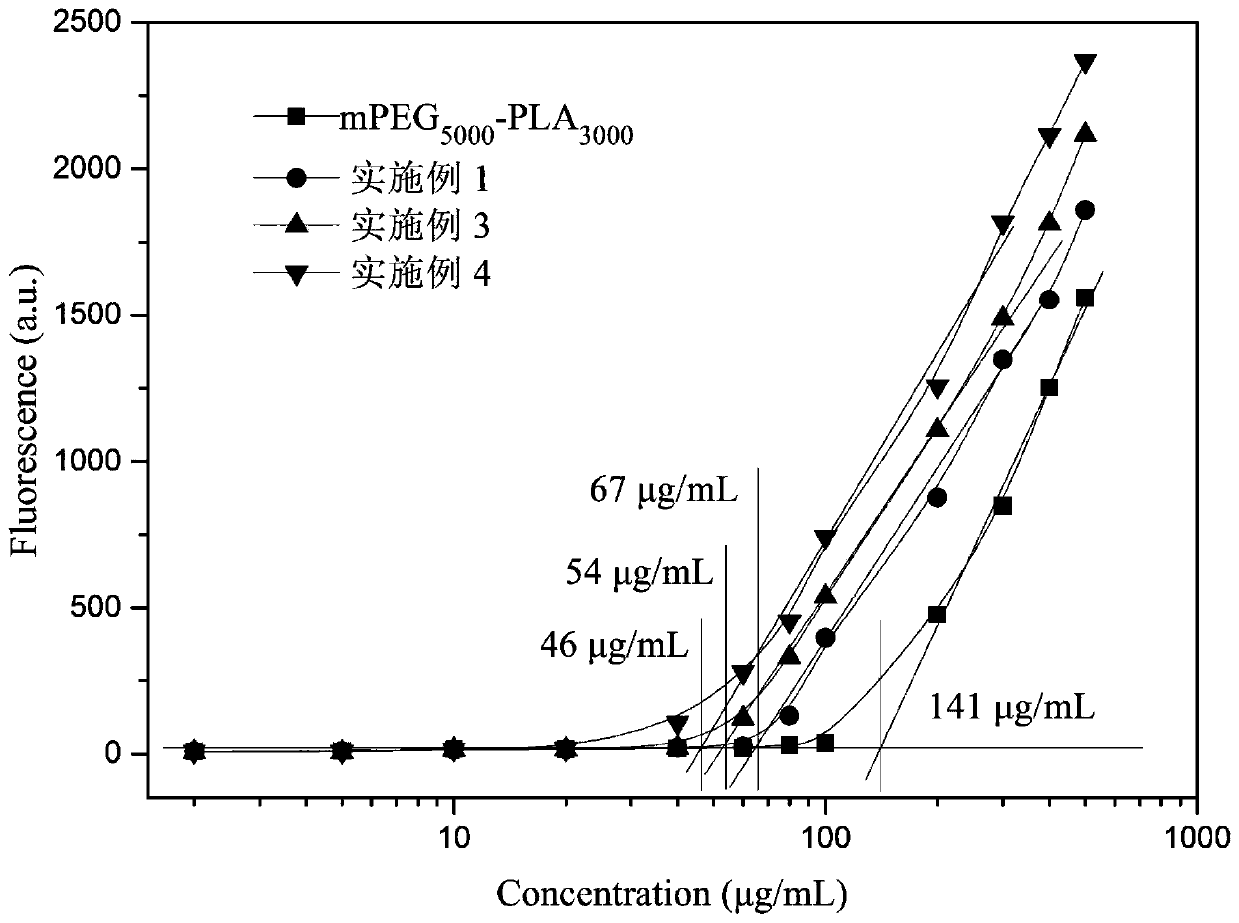
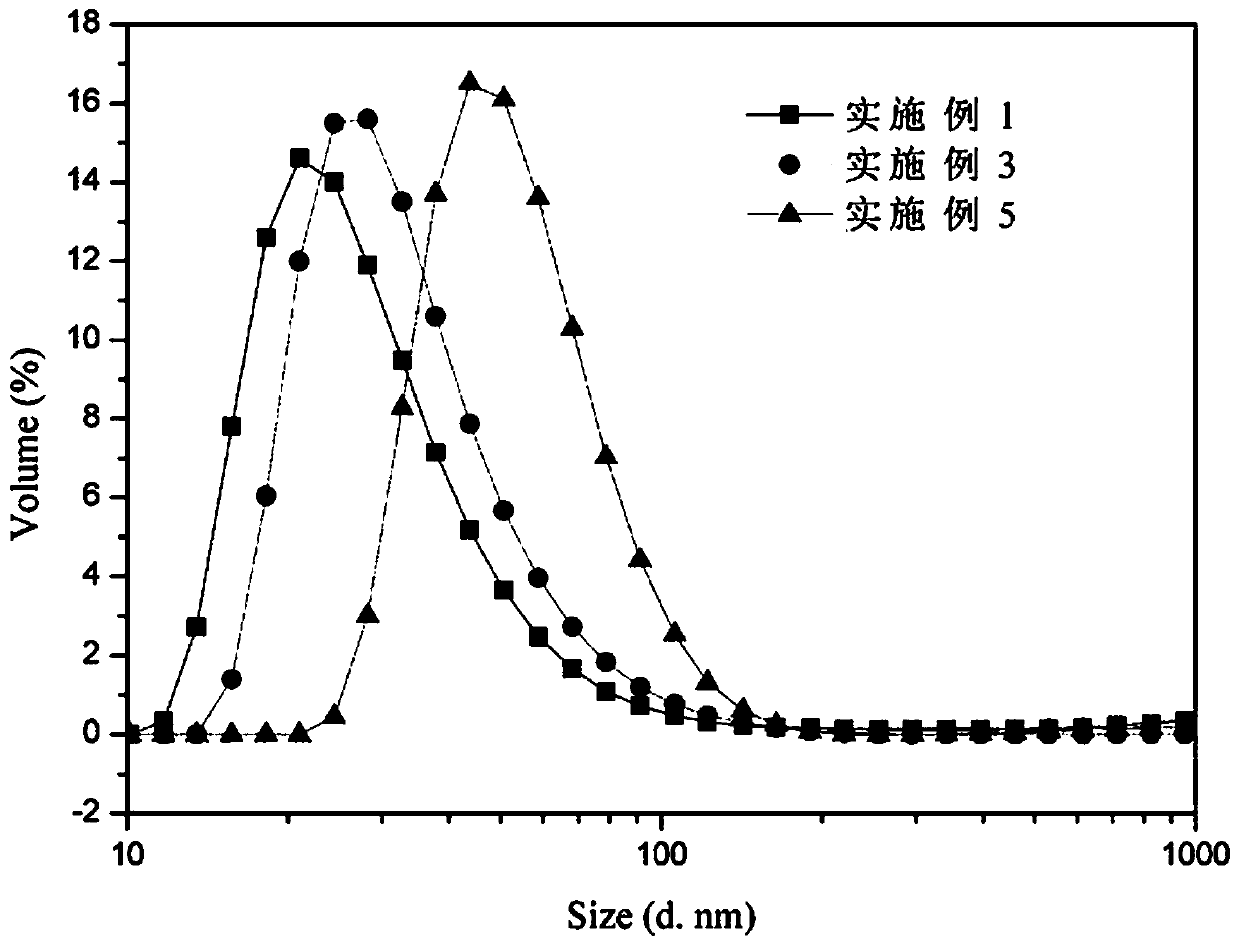
Examples
Embodiment 1
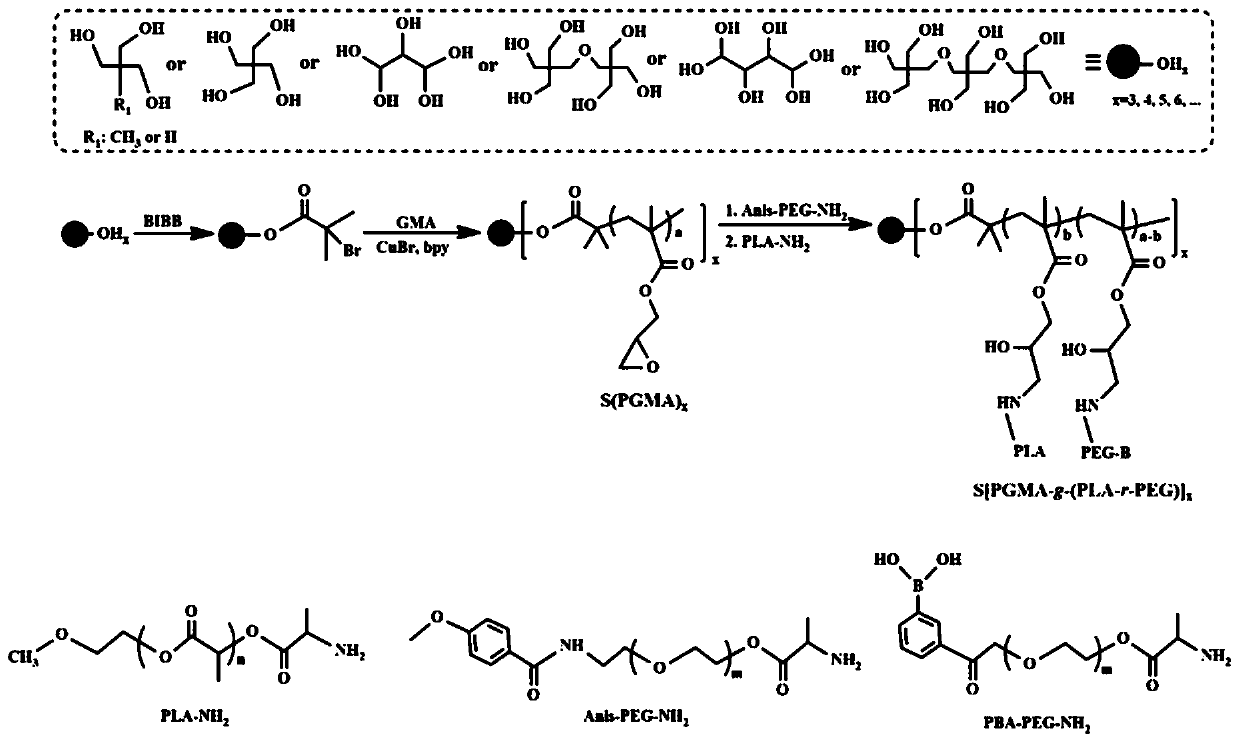
[0036] A preparation method of star-shaped amphiphilic polymer micelle nanomedicine with active tumor targeting:
[0037] (1) Preparation of ATRP-initiated monomers: add 1.35 g of trimethylolpropane, 3 g of dehydrated triethylamine and 100 mL of dichloromethane into a round-bottomed flask, cool to 0°C, and dropwise add 14 g of 2-bromoiso Butyryl bromide, the dropwise addition time was controlled to 2h, after the dropwise addition, the reaction was continued at 0°C for 2h, and then the temperature was slowly raised to 30°C for 48h. After the reaction, the product was washed three times with saturated sodium bicarbonate solution, saturated brine and deionized water respectively, and the organic phase was dried with anhydrous magnesium sulfate, and the ATRP initiating monomer (S-Br) was obtained by rotary evaporation and drying. 3 ).
[0038] (2) Star polymer [S(PGA) 3 ]: 0.058 g ATRP initiating monomer (S-Br 3 ) and 0.43 g of glycidyl methacrylate (GMA) were dissolved in 20 m...
Embodiment 2
[0042] A preparation method of star-shaped amphiphilic polymer micelle nanomedicine with active tumor targeting:
[0043] (1) Preparation of ATRP-initiated monomers: add 1.35 g of trimethylolpropane, 3 g of dehydrated triethylamine and 100 mL of dichloromethane into a round-bottomed flask, cool to 0°C, and dropwise add 14 g of 2-bromoiso Butyryl bromide, the dropwise addition time was controlled to 2h, after the dropwise addition, the reaction was continued at 0°C for 2h, and then the temperature was slowly raised to 30°C for 48h. After the reaction, the product was washed three times with saturated sodium bicarbonate solution, saturated brine and deionized water respectively, and the organic phase was dried with anhydrous magnesium sulfate, and the ATRP initiating monomer (S-Br) was obtained by rotary evaporation and drying. 3 ).
[0044] (2) Star polymer [S(PGA) 3 ]: 0.058 g ATRP initiating monomer (S-Br 3 ) and 0.85 g of glycidyl methacrylate (GMA) were dissolved in 20 m...
Embodiment 3
[0048] A preparation method of star-shaped amphiphilic polymer micelle nanomedicine with active tumor targeting:
[0049] (1) ATRP (S-Br 4 ) to initiate monomer preparation: 1.36g of pentaerythritol, 4g of dehydrated triethylamine and 100mL of dichloromethane were added to a round-bottomed flask, cooled to 0°C, and 18.4g of 2-bromoisobutyryl bromide was added dropwise. The addition time was controlled to 2h, after the dropwise addition, the reaction was continued at 0°C for 2h, and then the temperature was slowly raised to 30°C for 48h. After the reaction, the product was washed three times with saturated sodium bicarbonate solution, saturated brine and deionized water respectively, and the organic phase was dried with anhydrous magnesium sulfate, and the ATRP initiating monomer was obtained by rotary evaporation and drying.
[0050] (2) Star polymer [S(PGA) 4 ]: 0.073 g ATRP initiating monomer (S-Br 4 ) and 0.56 g of glycidyl methacrylate (GMA) were dissolved in 20 mL of a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com