Process method for preparing maleic anhydride from n-butane by oxidation
A process method, technology of n-butane, applied in the field of reaction process of n-butane oxidation to maleic anhydride, can solve the problems of reducing effective volume of reactor, increasing product yield, reducing production efficiency, etc. High anhydride yield and the effect of overcoming temperature drop
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] (2) Preparation of vanadium phosphorus oxygen catalyst
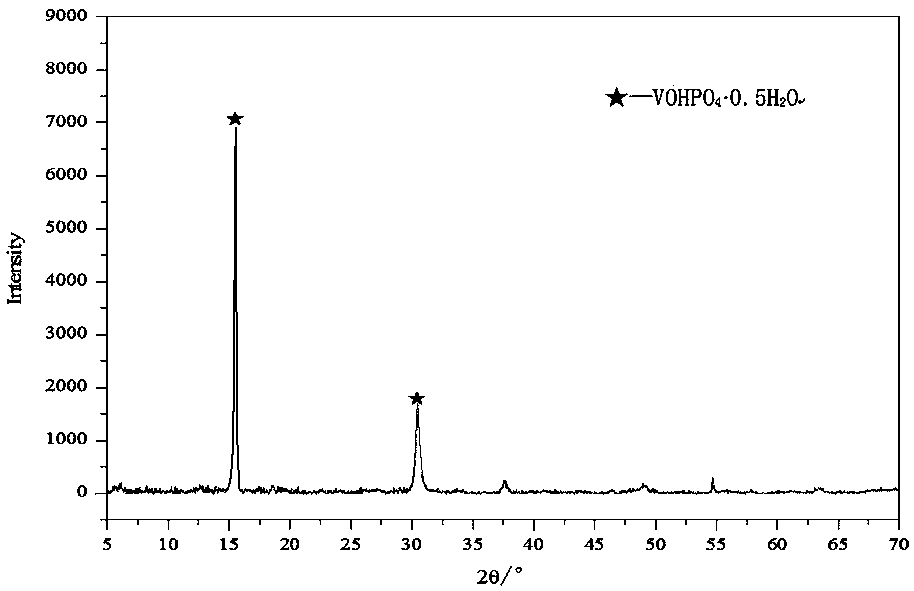
[0048] The vanadium phosphorus oxide obtained in step (1) is first shaped, and the shape of the obtained vanadium phosphorus oxygen catalyst can be a compressed tablet, a spherical shape, an extruded rod, etc., and the phase of the obtained catalyst precursor is (VOHPO 4 0.5H 2 o).
[0049] (3) Activation treatment of vanadium phosphorus oxygen catalyst
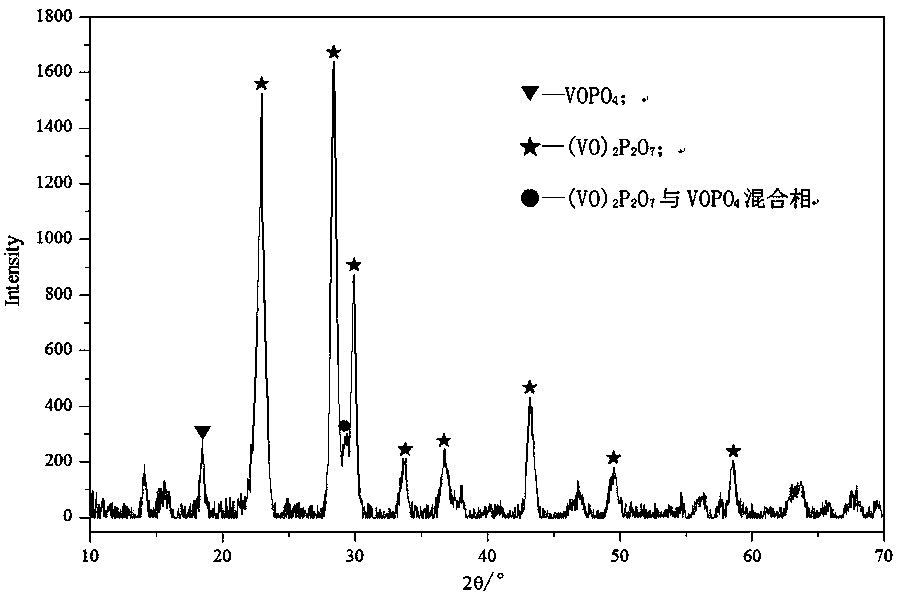
[0050] The shaped catalyst precursor in step (2) is activated in an atmosphere of nitrogen or inert gas, or in an atmosphere of n-butane / air mixture, to obtain an activated vanadium-phosphorus-oxygen catalyst.
[0051] Wherein in step (3), the activation temperature is generally 350-450°C, preferably 375-425°C; the activation time is generally 5-40 hours, preferably 12-20 hours. The volume space velocity of nitrogen, inert gas or n-butane / air mixed atmosphere is generally 100-2000h -1 , preferably 500~1000h -1 .
[0052] In the present invention, the prepar...
Embodiment 1
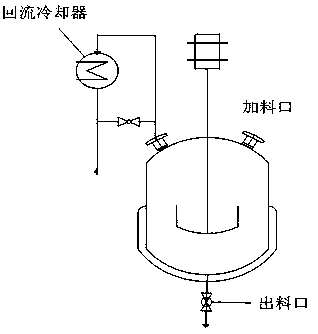
[0057] exist figure 1 Add 442.95 kg of isobutanol, 29.53 kg of vanadium pentoxide, 0.3 kg of ferric nitrate hexahydrate, and 0.5 kg of zirconium nitrate into the reaction kettle shown with a stirring device and a reflux condensing device. High reaction temperature and kept at 100±2°C, carry out reflux reaction, keep reflux time for 4 hours, then add 47.50kg of phosphoric acid with a concentration of 85%, the phosphorus / vanadium molar ratio is 1.27, continue to reflux for 8 hours, and the reaction ends. After the reaction solution was cooled to room temperature, it was vacuum filtered, and the filter cake was rinsed with a small amount of isobutanol three times, then the filter cake was placed in an enamel tray and dried naturally at room temperature, dried in an oven at 100°C for 8 hours, and finally dried in a muffle furnace. Calcined at 250°C for 5 hours to obtain a dark brown catalyst precursor.
[0058] Add the catalyst precursor prepared above to graphite powder with a m...
Embodiment 2
[0062] exist figure 1 Add 442.95 kg of isobutanol, 29.53 kg of vanadium pentoxide, 0.3 kg of ferric nitrate hexahydrate, and 0.5 kg of zirconium nitrate into the reaction kettle shown with a stirring device and a reflux condensing device. High reaction temperature and kept at 100±2°C, carry out reflux reaction, keep reflux time for 4 hours, then add 44.13kg of phosphoric acid with a concentration of 85%, the phosphorus / vanadium molar ratio is 1.18, continue to reflux for 8 hours, and the reaction ends. After the reaction solution was cooled to room temperature, it was vacuum filtered, and the filter cake was rinsed three times with a small amount of isobutanol to complete the reaction. The filter cake was put into an enamel dish and air-dried at room temperature, dried in an oven at 100°C for 8 hours, and finally calcined in a muffle furnace at 250°C for 5 hours to obtain a dark brown catalyst precursor.
[0063] Add the catalyst precursor prepared above to graphite powder wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com