A kind of thermophilic fungus cutinase and its coding gene and application
A protein and amino acid technology, applied in applications, fungi, genetic engineering, etc., can solve the problem of low expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1, expression of C. camphorii cutinase in recombinant Pichia pastoris
[0057] 1. Construction of recombinant bacteria
[0058] Using the cDNA of C. camphorii cutinase as a template, according to the reported fungal cutinase amino acid sequence, use the online software Block Maker (http: / / blocks.fhcrc.org / blocks / blockmkr / make_blocks.html) to search for the conserved region, Then use the online primer design software CODEHOP (http: / / blocks.fhcrc.org / codehop.html) to design degenerate primers, upstream primer McCutEcoRIF: 5'-tgcga GAATTC TCCCCAGTTGCAGTGGAGA-3' (the underline indicates the EcoRI restriction site, and the sequence after the underline is the same as the 49th-67th position in Sequence 1 in the sequence listing) and the downstream primer McCutNotIR: tgcga GCGGCCGC TTACGAGAGTCTATCCTCAAGCC (the underline indicates the Not I restriction site, and the sequence after the underline matches the 626th to 648th positions in Sequence 1 in the Sequence List...
Embodiment 2
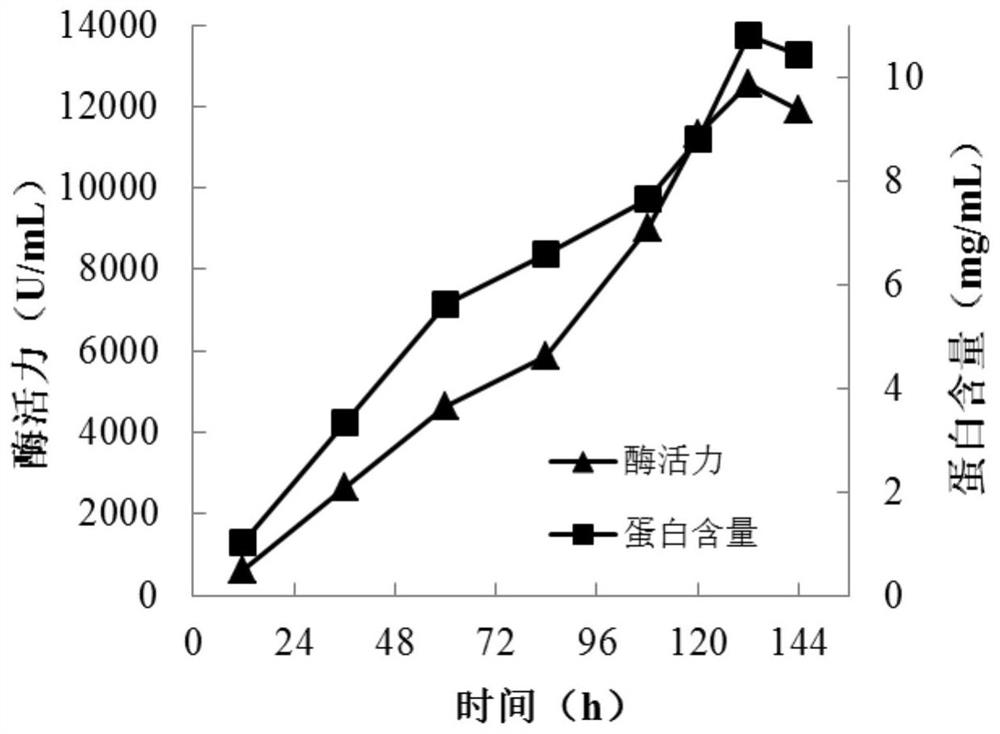
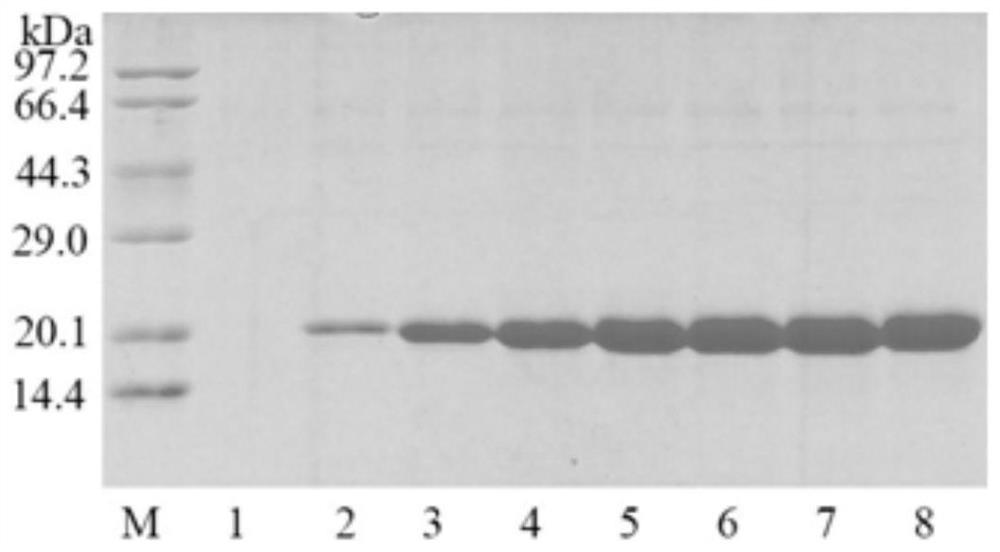
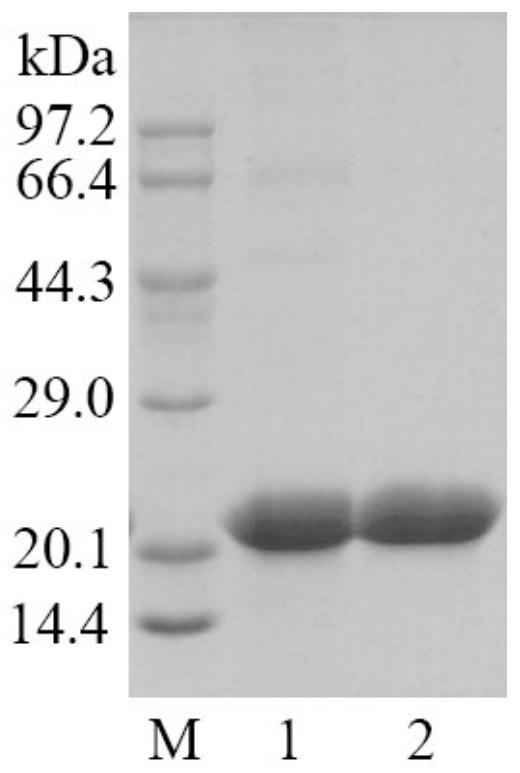
[0069] Embodiment 2, purification and enzymatic properties of cutinase
[0070] 1. Definition and determination method of cutinase enzyme activity
[0071] The enzymatic activity of cutinase was determined with reference to the method of Xu et al. (Xu et al., Characterization of anacidic cold-adapted cutinase from Thielavia terrestris and its application flavor ester synthesis. Food Chemistry, 2015, 188: 439-445). Add 50 μL of appropriately diluted cutinase solution to 400 μL of 50 mM Tris-HCl buffer (pH 8.0), preheat in a 45°C water bath for 2 min, add 50 μL of 20 mM p-nitrophenylbutyrate in isopropanol and react for 10 min. After the reaction was completed, 500 μL of 300 mM phosphate buffer (pH 7.0) containing 5% (w / v) SDS (sodium dodecyl sulfate) was added. Finally, absorbance was measured at 410 nm. Enzyme activity unit (U) is defined as: under the above conditions, the amount of cutinase required to produce 1 μmol of p-nitrophenol per minute. Specific enzyme activity i...
Embodiment 3
[0083] Embodiment 3, cutinase hydrolysis butter
[0084] Hydrolysis of butter with cutinase: Mix 1g of butter with 1g of 50mM phosphate buffer (pH 8.0), add 1500-4000U / g of cutinase, and hydrolyze at 45°C and 200rpm. After 12 hours of hydrolysis, add 15 mL of ether: ethanol (2:1, v / v) mixed solvent, and then titrate with 0.1 mol / L KOH solution to determine the acid value.
[0085] Gas chromatography-mass spectrometry (GC-MS) detection of reaction products: After hydrolysis, take the upper oil sample, add n-hexane to dilute, mix well, and perform GC-MS detection. The chromatographic column DB-WAX (30m×0.25mm×0.25μm) was used, the injection volume was 1μL, the split ratio was 1:20, He was used as the carrier gas, and the flow rate was 1.5mL / min; 2min, 5°C / min to 210°C, hold for 5min; GC / MS interface temperature 250°C, EI ion source, ion source temperature 220°C, electron energy 70eV, mass spectrometry scanning range 33-450aum.
[0086] When the amount of cutinase added was 300...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com