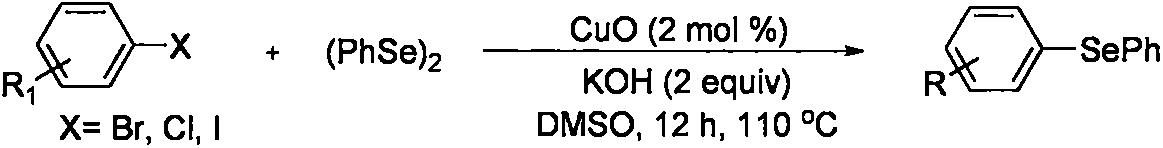
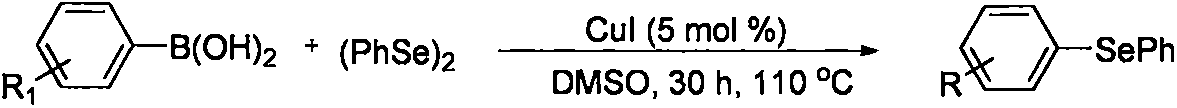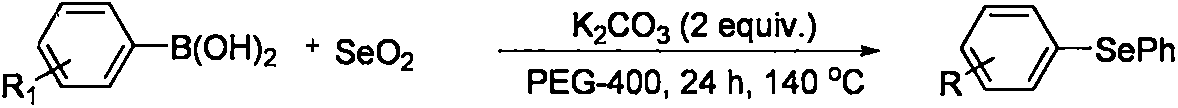2-(2,4,6-trimethylbenzeneseleno)-5-methylbenzoxazole compound and preparation method thereof
A technology of methylbenzoxazole and trimethylbenzene, which is applied in the field of organic compound synthesis, can solve the problems of increased synthesis cost, environmental pollution, and large quantities, and achieves the effects of simple operation, simple post-processing operation, and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Synthesis of 2-(2,4,6-trimethylphenylselenyl)-5-methylbenzoxazole
[0050]
[0051] At room temperature, 2,4,6-trimethyliodobenzene (1.2mmol, 3equiv), elemental selenium (1.2mmol, 3equiv), 5-methylbenzoxazole (0.4mmol, 1equiv), Cu(OAc ) 2 (0.04mmol), ammonium bicarbonate (1.2mmol, 3equiv) were added to the reaction tube, then filled with nitrogen, and replaced three times, under the nitrogen reaction environment, then added 2mL DMF reaction solvent, stirred at 140 ° C reaction temperature for 24h . After the end of the reaction was monitored by thin-layer chromatography, the reaction mixture was cooled, then diluted with ethyl acetate, the diluted solution was transferred to a separatory funnel, extracted with saturated brine, the aqueous phase and the organic phase were separated, and then diluted with acetic acid Extract the aqueous phase 3 times with ethyl ether, combine the organic phases, add 5g of anhydrous sodium sulfate, stand still for 30min, wash the filt...
Embodiment 2
[0058] Scaled-up Synthesis of 2-(2,4,6-Trimethylphenylselenyl)-5-methylbenzoxazole
[0059]
[0060] At room temperature, 2,4,6-trimethyliodobenzene (12mmol, 3equiv), elemental selenium (12mmol, 3equiv), 5-methylbenzoxazole (4mmol, 1equiv), Cu(OAc) 2 (0.4mmol), ammonium bicarbonate (12mmol, 3equiv) were added to the reaction tube, then filled with nitrogen, and replaced three times, under nitrogen reaction environment, then added 20mL DMF reaction solvent, stirred at 140 ° C reaction temperature for 24h. After the end of the reaction was monitored by thin-layer chromatography, the reaction mixture was cooled, then diluted with ethyl acetate, the diluted solution was transferred to a separatory funnel, extracted with saturated brine, the aqueous phase and the organic phase were separated, and then diluted with acetic acid Extract the aqueous phase 3 times with ethyl ester, combine the organic phases, add 25g of anhydrous sodium sulfate, stand still for 30min, wash the filter...
Embodiment 3-14
[0063] Except that the catalyzer copper acetate therein is replaced by the following copper catalyst respectively, in the same manner as Example 1 with the highest product yield, embodiments 3-14 have been implemented respectively, the yield of the copper compound used and the corresponding product As shown in Table 1 below.
[0064] Table 1
[0065]
[0066]
[0067] As can be seen from Table 1 above, when other copper compounds are used, the product yields are greatly reduced. This proves that the catalyst copper acetate used in the present invention has efficient catalytic performance for this reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com