Polyionic liquid gel with nanopores and method thereof
A technology of polyionic liquids and nanopores, applied in chemical instruments and methods, organic chemistry, alkali metal oxides/hydroxides, etc., can solve problems such as poor adsorption capacity and no network, and achieve increased utilization and excellent performance The effect of adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0045] Experimental example 1 terminal group has the synthetic method of the A monomer of imidazole
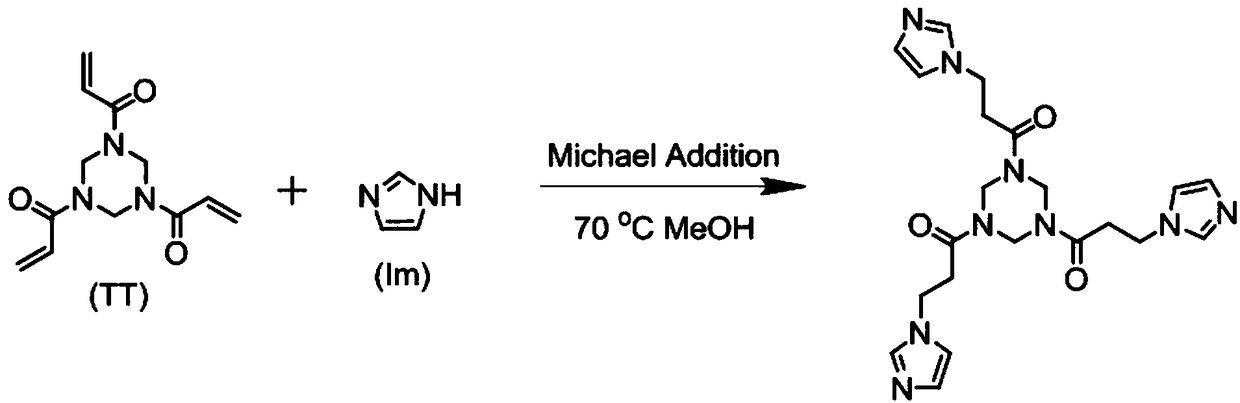
[0046] Such as figure 2 Shown is the synthetic route diagram of A monomer of Experimental Example 1 of the present invention, through 1,3,5-triacryloyl hexahydro-1,3,5-triazine (TT) and imidazole (Im) Michael addition The reaction is prepared, and the specific technical scheme is as follows:
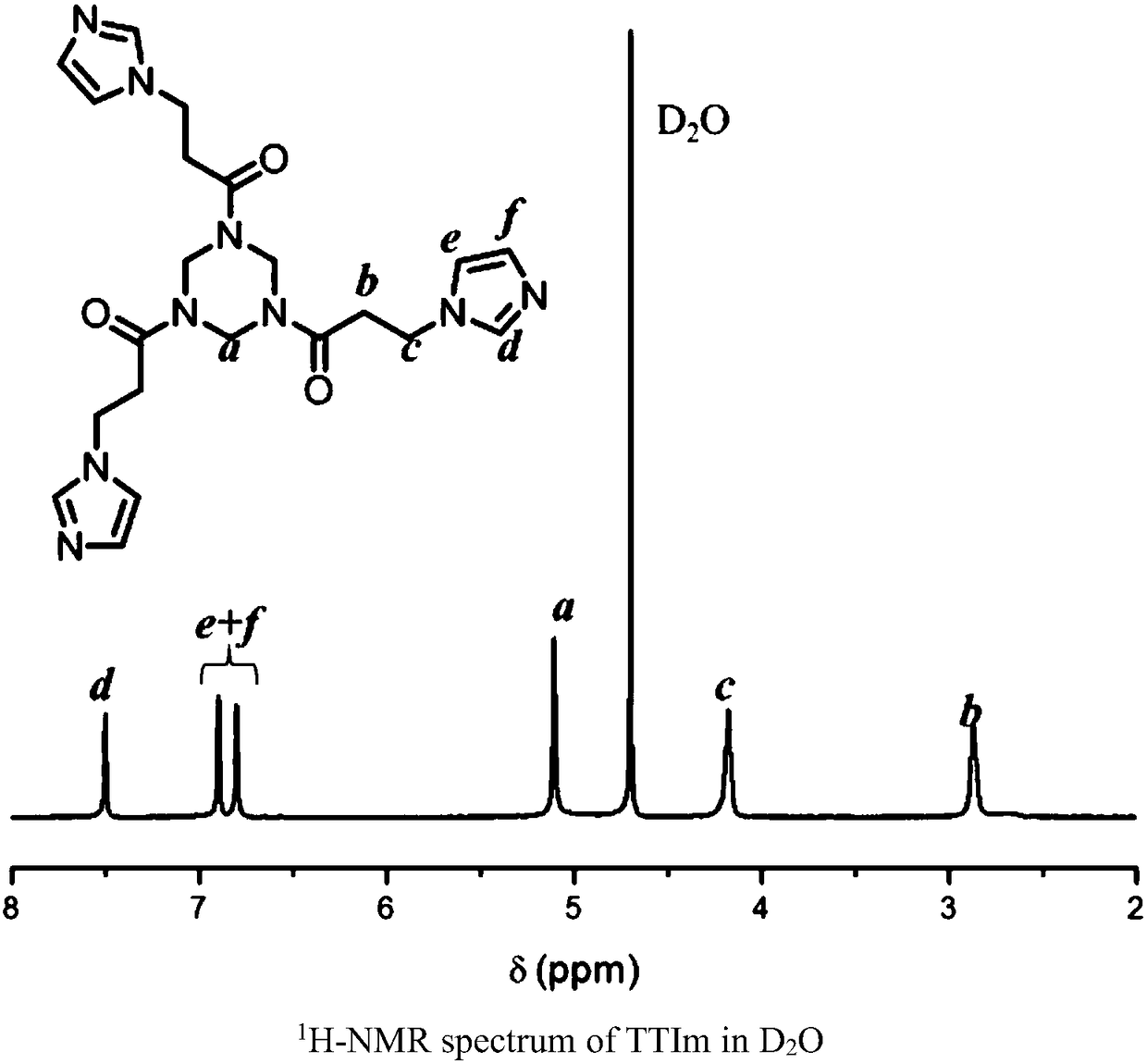
[0047] TT (5g, 20mmol) and Im (10g, 106mmol) were added to 30mL of anhydrous methanol and refluxed for 24h. After the reaction, the reaction system was precipitated in a mixed solvent of ethyl acetate / tetrahydrofuran (1:1 by volume). After dissolving-precipitation (methanol-ethyl acetate / tetrahydrofuran), washing twice, and filtering, the resulting precipitate was vacuum-dried to constant weight at room temperature to obtain A monomer (TT Im), such as image 3 Shown is the NMR spectrum of A monomer prepared in Experimental Example 1 of the present invention.
experiment example 2
[0048] Experimental example 2 end group has the synthetic method of chlorine or bromine B monomer
[0049] Such as image 3 Shown is the synthesis route diagram of the B monomer of Experimental Example 2 of the present invention, with polyoxyethylene glyceryl ether (B-PEG-OH, that is, compound C) and chloroacetic acid (CAA) or bromoacetic acid (BAA) as typical Reaction, the technical scheme is as follows:
[0050] B-PEG-OH (10g, 10mmol), CAA (4.25g, 45mmol) or BAA (6.25g, 45mmol), p-TSA (0.11g, 0.6mmol) (p-TSA, p-toluenesulfonic acid) and 200mL of toluene was added to the Dean-Stark apparatus, heated to 120°C, and refluxed for 12h. After the reaction, the toluene was evaporated, and the crude product was dissolved in dichloromethane, followed by washing with 2% Na 2 CO 3 Wash 3 times, wash 2 times with 5% NaCl, and wash with pure water until the pH of the aqueous layer is neutral. with anhydrous MgSO 4 Dry the organic layer, filter, remove the solvent under reduced pressur...
experiment example 3
[0051] The preparation method of experimental example 3 imidazolium salt type hyperbranched polyionic liquid
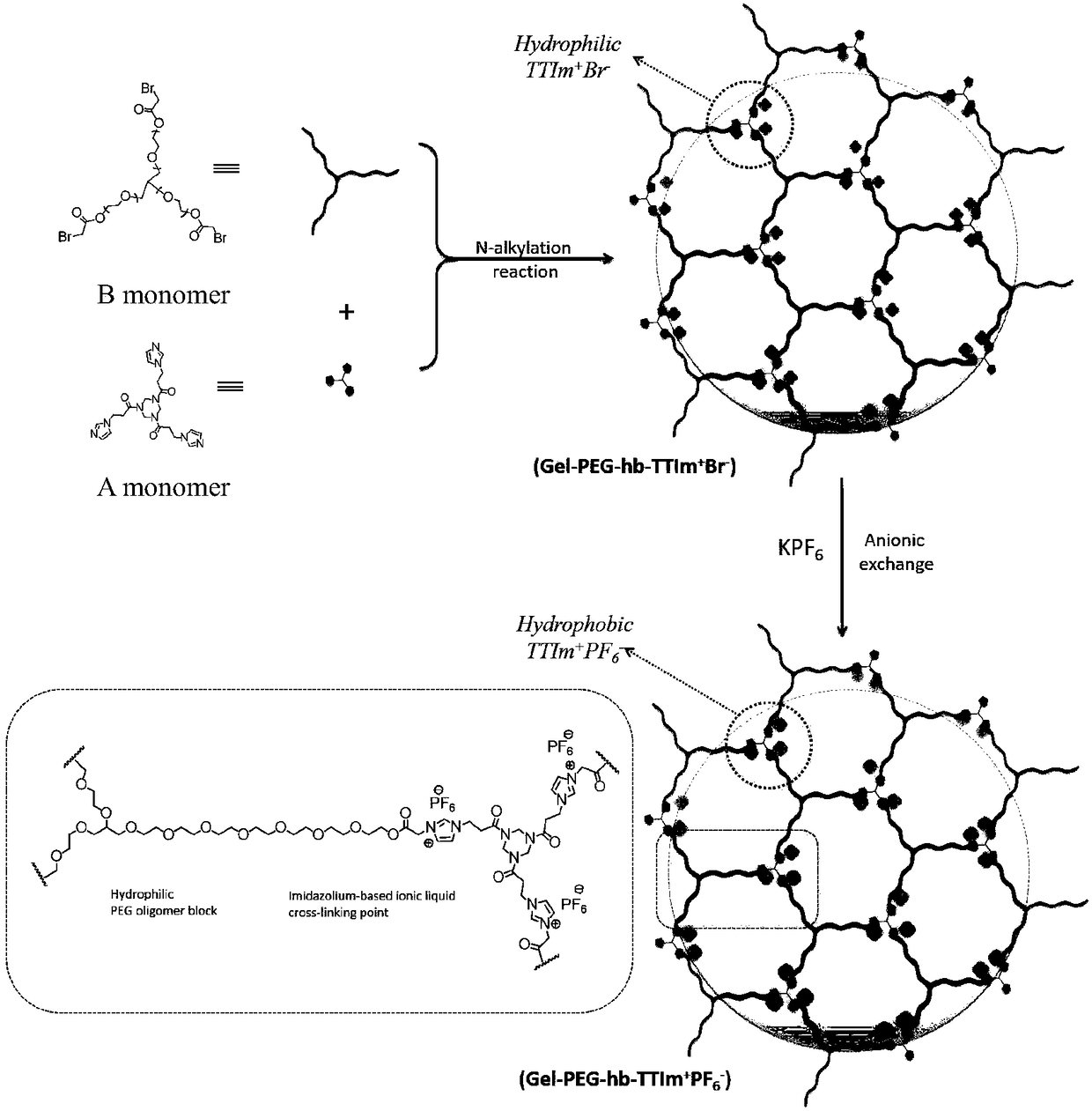
[0052] Add 660mg A monomer and 660mg B monomer (equal molar ratio of A and B reacting functional groups) and different volumes of dry DMF into the reagent bottle (A monomer and B monomer with bromine at the end group get samples 1#~6# ), after stirring to dissolve, take out the rotor. Seal the reaction bottle and place it in an oil bath at 60-80°C for 14-24 hours. After the reaction, the reaction bottle was taken out from the oil bath, and the system was cooled to room temperature, and the sample was taken out and sliced. Soak the removed gel in deionized water for 2 days (during which the water was changed 3 times a day) to wash away unreacted monomers and DMF. Then, the gel piece was pulled out, placed in an ethanol solution and soaked for one day (during which, the ethanol was changed 3 times a day), to replace the water in the gel network, the gel piece was pull...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| gel fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com