Method for producing n-propylbenzene by using benzene and isopropylbenzene
A technology for cumene and n-propyl benzene, applied in the field of producing n-propyl benzene, can solve problems such as being unsuitable for industrial production, cumbersome reaction steps and the like, and achieve the effects of low production cost and simple technological process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
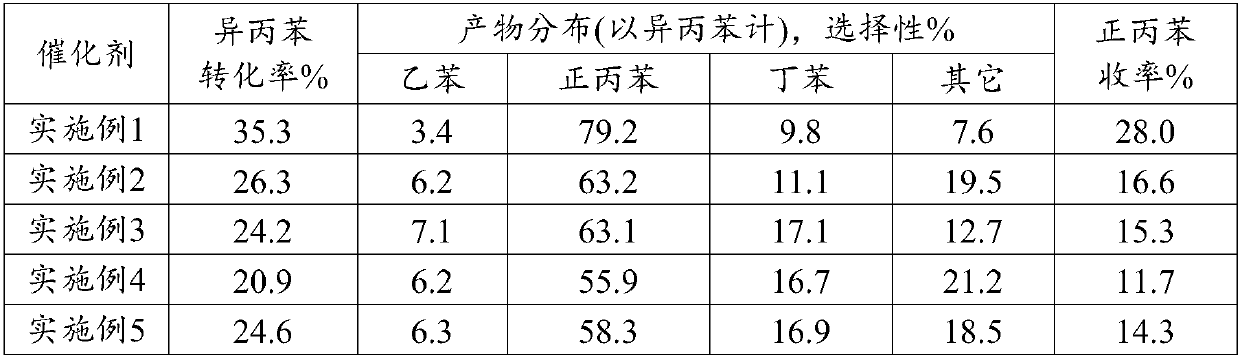
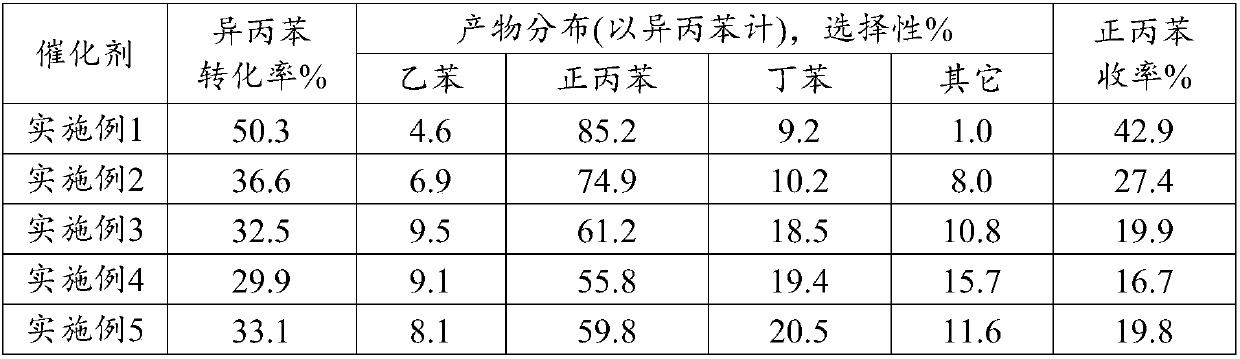
Embodiment 1
[0018] Synthesis of ZSM-5 molecular sieves with MFI topology.
[0019] First prepare colloidal seeds, 710.3g H 2 O, 13.8g NaOH and 117.0g TPAOH (20% solution) solution are fully dissolved and mixed until uniform, and then 158.9g silicic acid is gradually added to the above solution under stirring, fully shaken at room temperature for 1h, and aged at 100°C 16h; 867.8g H 2 O, 8.8g NaOH and 10.3g sodium aluminate are fully mixed and dissolved, 113.1g silicic acid is gradually added to the above mixed solution under full stirring and shaken violently for 1h, and 50g of the prepared colloidal seed crystals are added to the above mixed solution Shake for 1 h, then transfer the material to a stainless steel reactor. After crystallization at 180°C for 40h, the crystalline product was filtered, washed and dried. The crystalline product obtained by XRD powder diffraction analysis is ZSM-5 molecular sieve, and its silicon-aluminum ratio is 12-13.5.
[0020] Take 50 g of dried powder ...
Embodiment 2
[0022] Synthesis of Y-type molecular sieves with FAU topology.
[0023] First prepare colloidal directing agent, 20.0g H 2 O, 4.0g NaOH and 2.1g sodium aluminate (20% solution) solution were fully dissolved and mixed until uniform, then 22.7g silicic acid was gradually added to the above solution under stirring, fully shaken at room temperature for 1h, and aged at room temperature overnight; 261.9g H 2 O, 0.28g NaOH and 20.6g sodium aluminate are fully mixed and dissolved, 284.8g silicic acid is gradually added to the above mixed solution under full stirring and shaken violently for 1h, and 50g of the prepared directing agent is added to the above mixed solution Shake in medium for 1h. The material was then transferred to a stainless steel reactor. After crystallization at 100°C for 10 h, the crystalline product was filtered, washed and dried. The crystalline product obtained by XRD powder diffraction analysis is Y-type molecular sieve.
[0024] Take 50 g of dried powder ...
Embodiment 3
[0026] Synthesis of Beta molecular sieves with BEA topology.
[0027] 600g 40wt.% silica sol, 38.9g sodium aluminate (alumina content 42wt.%), 70.6g 25wt.% tetraethylammonium hydroxide (TEAOH), 5.0g diethylamine (DEA), 168.0g tetraethyl Ammonium bromide (TEABr), 16.0g of sodium hydroxide, 136.0g of 25wt.% ammonium hydroxide, and 925.1g of water were mixed and stirred evenly at room temperature. Then transferred to a stainless steel autoclave, the crystallization temperature was 155°C, and reacted for 50 hours. After the reaction, the crystalline product was filtered, washed and dried. The crystalline product obtained by XRD powder diffraction analysis is Beta zeolite.
[0028] Take 50 g of dried powder sample, exchange 4 times with 1M ammonium nitrate, filter and dry. After that, fully mix with 20g alumina, and add 5wt% nitric acid to knead, extrude and form into The strips were dried at 120°C for 12 hours and calcined at 500°C for 6 hours to prepare the required cataly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com