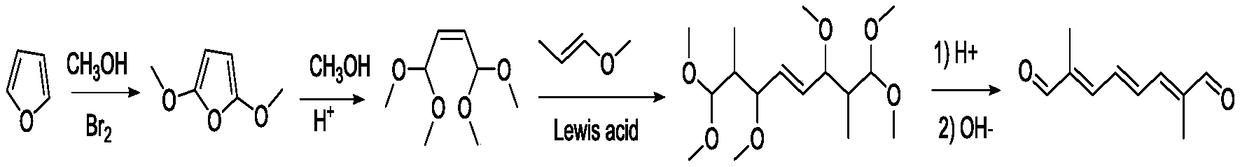
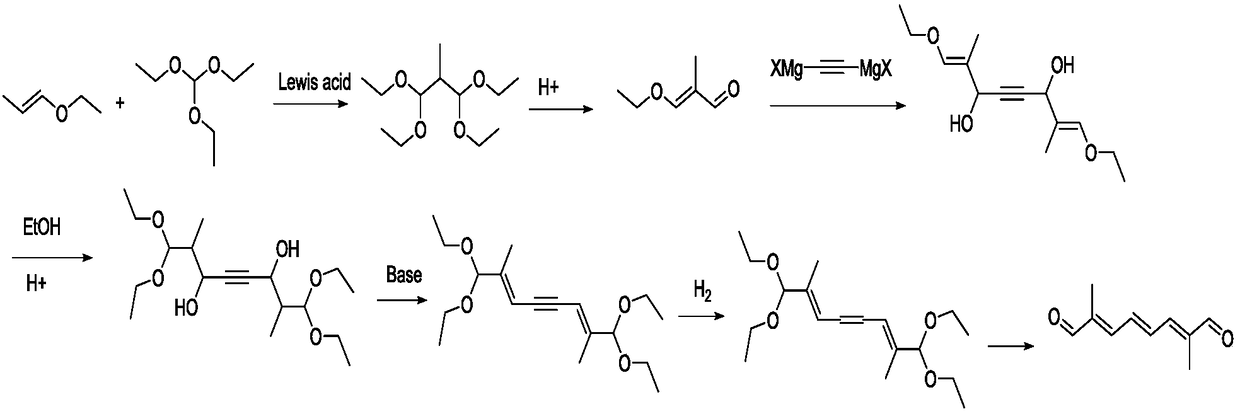
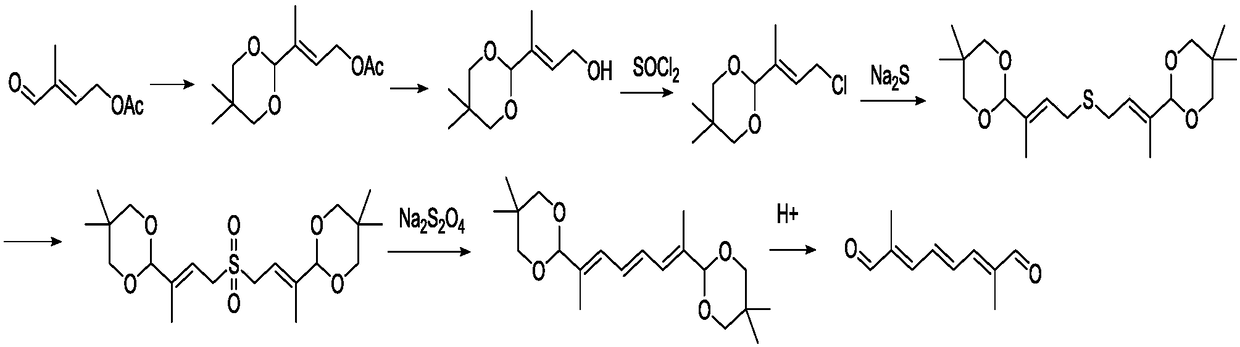
Preparation method of 2,7-dimethyl-2,4,6-octatriene-1,8-dial
A technology of octatriene and dimethyl, which is applied in the field of preparation of 2,7-dimethyl-2,4,6-octatriene-1,8-dialdehyde, which can solve the problem of long reaction route and high three wastes , poor selectivity and other problems, to achieve the effect of easy industrial production and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of embodiment 1 compound V
[0040] Weigh 100g of tetrahydrofuran and 24g (1mol) of magnesium flakes in a 2L three-necked flask, then add 0.1g of iodine to initiate the reaction, then add dropwise 106.5g (0.5mol) of 1,4-dibromo-2-butene and 500g of For the mixed solution of tetrahydrofuran, control the internal temperature of the reaction at 55-60 ° C, drop it completely in about 2 hours, continue the insulation reaction for 1 hour after the drop is complete, and then add 44.1 g of ethylene oxide dropwise to the reaction solution, and control the reaction temperature not to exceed 60 ℃, 2h dropwise addition is complete, after the dropwise addition is complete, continue the insulation reaction for 2h, slowly pour the reaction solution into 300g 5wt% hydrochloric acid for acidolysis, the acidolysis temperature does not exceed 10°C, continue to stir for 30min after the addition is complete, and separate the liquid. Tetrahydrofuran was removed under reduced ...
Embodiment 2
[0041] The preparation of embodiment 2 compound V
[0042] Weigh 100g of tetrahydrofuran and 25.2g (1.05mol) of magnesium flakes in a 2L three-necked flask, then add 0.1g of iodine to initiate the reaction, and then dropwise add 106.5g (0.5mol) of 1,4-dibromo-2-butene into the system Mixed solution with 500g tetrahydrofuran, control the internal temperature of the reaction at 55-60°C, and drop it completely in about 3 hours. When the temperature exceeds 60°C, the dropwise addition is complete in about 3 hours. After the dropwise addition is complete, continue to keep warm for 1 hour. Slowly pour the reaction solution into 300g of 5% hydrochloric acid for acidolysis. The acidolysis temperature does not exceed 10°C. After the addition is complete, continue to stir for 30 minutes. Liquid separation, removal of THF under reduced pressure (absolute pressure 1000Pa, temperature 45°C), and then vacuum oil pump drying (vacuum absolute pressure 30Pa, drying temperature 50°C), to obtain...
Embodiment 3
[0043] The preparation of embodiment 3 compound VI
[0044] Weigh 28.8g (0.2mol) of compound V prepared in Example 1, 200g of dichloromethane, 0.3g (0.002mol) of 2,2,6,6-tetramethylpiperidine oxide in a 500ml three-necked flask, at 20°C Under stirring, oxygen gas is blown into the system at a speed of 100ml / min. The reaction process is detected by gas phase, and the reaction of compound V is complete in about 5h. In the system, 100g of water is added to terminate the reaction, liquid separation, and the organic phase solvent (absolute Pressure 1000Pa, temperature 30°C), and then vacuum oil pump drying (vacuum absolute pressure 20Pa, drying temperature 50°C), to obtain 25.6g of compound VI, product gas phase purity 99.5%, yield 91.4%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com