Preparation method of covalent light emitting organic semiconductor polymer nanofiber with triazine-like structure and application of covalent light emitting organic semiconductor polymer nanofiber in photocatalytic hydrogen production
A technology of organic semiconductors and nanofibers, which is applied in the field of covalent triazine-like light-emitting organic semiconductor polymer nanofiber materials to achieve excellent fluorescent properties, excellent electron transport capabilities, and stable luminescent properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
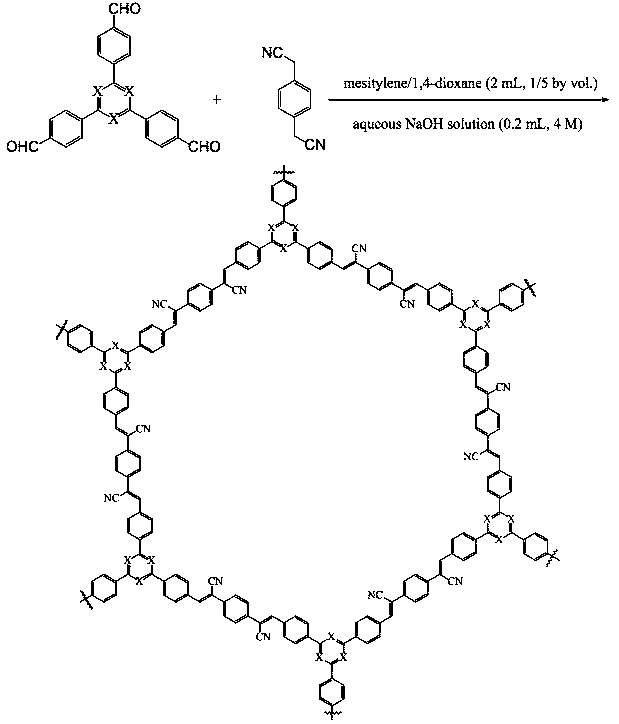
[0032] Add 25.0 mg 4-[3,5-bis(4-formylphenyl)phenyl]benzaldehyde (0.064 mmol), 15.0 mg 1,4-benzenediacetonitrile (0.096 mmol) to a 5 ml reaction flask Then add 0.334 ml of mesitylene and 1.766 ml of dioxane for ultrasonic vibration. Add 0.2 mL of 4 M sodium hydroxide aqueous solution dropwise to the reaction eggplant bottle. After the addition, the system was frozen with liquid nitrogen, the pressure in the glass bottle was reduced to below 10 mbar under the vacuum adsorption line system, the temperature was raised to room temperature and then filled with argon, and the operation was repeated three times. After the system was raised to room temperature, it was placed in an oven at 90°C to react for 3 days. After cooling down to room temperature, it was washed with water three times, and then washed with dichloromethane, DMF and acetone respectively. The solid was put into a Soxhlet extractor, washed with THF for 2 days, and then dried in a vacuum oven at 110°C for 12 hours t...
Embodiment 2
[0034]Add 6.3 mg 2,4,6-tris(4-formylphenyl)-1,3,5-triazine (0.016 mmol), 18.8 mg 4-[3,5-di (4-formylphenyl)phenyl]benzaldehyde (0.048 mmol), 15.0 mg 1,4-benzenediacetonitrile (0.096 mmol) were added, and then 0.334 ml of mesitylene and 1.766 ml of dioxane were ultrasonically oscillated. Add 0.2 mL of 4 M sodium hydroxide aqueous solution dropwise to the reaction eggplant bottle. After the addition, the system was frozen with liquid nitrogen, the pressure in the glass bottle was reduced to below 10 mbar under the vacuum adsorption line system, the temperature was raised to room temperature and then filled with argon, and the operation was repeated three times. After the system was warmed to room temperature, it was placed in an oven at 90 °C for 3 days. After cooling down to room temperature, it was washed with water three times, and then washed with dichloromethane, DMF and acetone respectively. Put the solid into a Soxhlet extractor, wash it with THF for 2 days, and then dr...
Embodiment 3
[0036] Add 12.6 mg 2,4,6-tris(4-formylphenyl)-1,3,5-triazine (0.032 mmol), 12.5 mg 4-[3,5-di (4-formylphenyl)phenyl]benzaldehyde (0.032 mmol), 15.0 mg 1,4-benzenediacetonitrile (0.096 mmol) were added followed by 0.334 ml mesitylene and 1.766 ml dioxane for ultrasonic vibration. Add 0.2 mL of 4 M sodium hydroxide aqueous solution dropwise to the reaction eggplant bottle. After the addition, the system was frozen with liquid nitrogen, the pressure in the glass bottle was reduced to below 10 mbar under the vacuum adsorption line system, the temperature was raised to room temperature and then filled with argon, and the operation was repeated three times. After the system was warmed to room temperature, it was placed in an oven at 90 °C for 3 days. After cooling down to room temperature, it was washed with water three times, and then washed with dichloromethane, DMF and acetone respectively. Put the solid into a Soxhlet extractor, wash it with THF for 2 days, and then dry it in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com