Folic acid targeted modified co-loaded doxorubicin hydrochloride and gambogic acid nanostructure lipid carrier preparation and preparation method thereof
A technology of nanostructured lipids and doxorubicin hydrochloride, which is applied in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., can solve the problem of the lack of specific selectivity of tumor cells and the difficulty in obtaining therapeutic effects. , tumor targeting is not obvious and other problems, to achieve the effect of improving bioavailability, improving water insolubility, and prolonging residence time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The invention provides a nanostructured lipid carrier preparation co-loaded with doxorubicin hydrochloride and gambogic acid for targeted modification of folic acid, comprising the following components:
[0048]
[0049] Preferably, the weight part of the doxorubicin hydrochloride is 0.05-0.5 part.
[0050] Preferably, the weight part of the gambogic acid is: 0.05-0.5 part.
[0051] Preferably, the weight portion of the phospholipid-polyethylene glycol-folic acid is 0.01-0.48 parts.
[0052] Preferably, the solid lipids are stearic acid, behenic acid, glyceryl behenate, glyceryl monostearate, glyceryl palmitostearate, glyceryl myristate, glyceryl monopalmitate and lauryl One or more of glycerides.
[0053] Preferably, the liquid lipid is one or more of caprylic capric macrogol glyceride, caprylic capric triglyceride, isopropyl palmitate, isopropyl myristate, soybean oil and oleic acid kind.
[0054]Preferably, the fat-soluble emulsifier is soybean lecithin and / or...
Embodiment 2
[0072] Accurately weigh a certain amount of doxorubicin hydrochloride, add 2 mL of deionized water to dissolve, and weigh 2 parts by weight of poloxamer, add it to 9 mL of deionized water, and dissolve in a water bath at 74°C. Under magnetic stirring, 0.01 parts by weight of fully dissolved doxorubicin hydrochloride aqueous solution was added to the poloxamer, and mixed uniformly to form an aqueous phase together. Weigh 0.01 parts by weight of gambogic acid and dissolve it in 2mL of absolute ethanol. After the dissolution is complete, add 0.1 parts by weight of soybean lecithin to mix the two evenly; weigh 0.01 parts by weight of DSPE-PEG-FA and dissolve them in In DMSO, add preparation simultaneously as organic phase; In addition, take 0.1 weight part of stearic acid as solid lipid, 0.1 weight part of isopropyl palmitate as liquid lipid, add 1mL chloroform to make it dissolve, and ethanol solution and The chloroform solutions were combined and mixed to form an oil phase. Kee...
Embodiment 3-11
[0074] Examples 3-11 prepared formulations according to the components in Table 2.
[0075] Table 2 each component of preparation and parts by weight thereof
[0076]
[0077]
[0078] In order to further understand the medicine, the inventors also conducted studies on the antitumor activity and tumor targeting properties of the preparation prepared by the present invention. The test methods and evaluations are as follows:
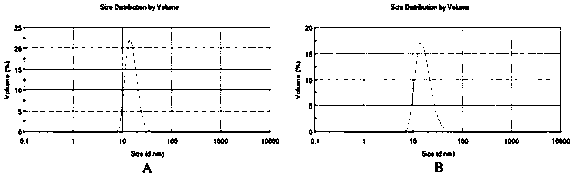
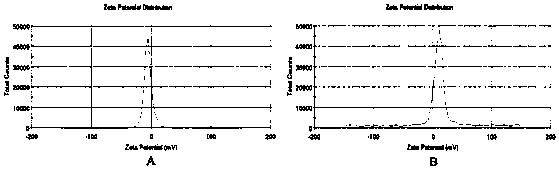
[0079] 1. Determination of particle size, polydispersity index (PDI) and zeta potential of nanostructured lipid carriers
[0080] Take an appropriate amount of the prepared doxorubicin hydrochloride / gambogic acid nano-structured lipid carrier and folic acid targeted modified doxorubicin hydrochloride / gambogic acid nano-lipid carrier solution, dilute to a suitable concentration with deionized water, and place in In the laser particle size analyzer, the particle size, distribution and Zeta potential value were measured at 25°C. The results are shown ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| electric potential / voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com