Platinum-non-precious metal alloy nanowire, and aqueous-phase synthesis method and application thereof
A non-precious metal and alloy nanotechnology, applied in the direction of nanotechnology, nanotechnology, metal processing equipment, etc., can solve the problems of affecting the catalytic performance of the catalyst, the reaction process is complicated, the reaction rate is fast, etc., and it is suitable for large-scale production and the preparation method is simple. , the effect of good catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
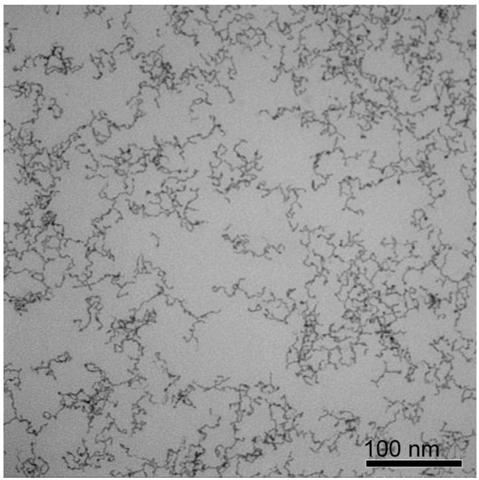
Embodiment 1
[0041] A water-phase synthesis method of platinum-nickel alloy nanowires, comprising the following steps:
[0042] (1) Preparation of platinum-nickel precursor solution
[0043] Add chloroplatinic acid, nickel chloride, sodium sulfite, and pH regulator into water so that the concentration of chloroplatinic acid and nickel chloride is 1.25×10 -5mol / L, the molar mass ratio of chloroplatinic acid to sodium sulfite is 1:6, adding a pH regulator to make the pH of the reaction solution 8, and standing at 30°C for 12 hours to obtain a platinum-nickel precursor solution.
[0044] (2) Preparation of ultrafine platinum-nickel alloy nanowires
[0045] The precursor concentration for preparing platinum-nickel metal is 1.25×10 -5 mol / L aqueous solution, adding vinylpyrrolidone and formic acid, so that the molar ratio of the precursor of platinum-nickel metal, vinylpyrrolidone, and formic acid is 1:100:40000 in sequence, and the obtained solution is moved to a hydrothermal reaction kettle...
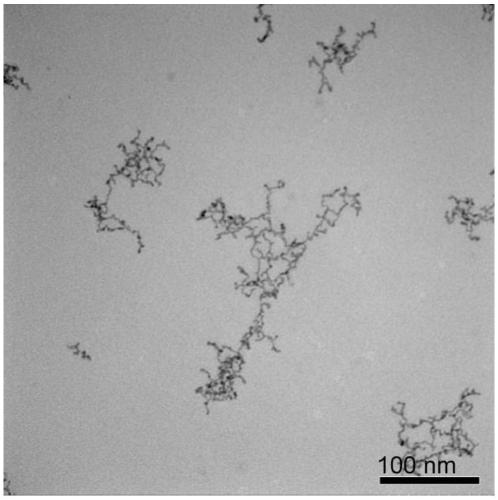
Embodiment 2
[0048] A water-phase synthesis method of platinum-cobalt alloy nanowires, comprising the following steps:
[0049] (1) Preparation of platinum-cobalt precursor solution
[0050] Add chloroplatinic acid, cobalt chloride, sodium sulfite, and pH regulator into water so that the concentration of chloroplatinic acid and cobalt chloride is 1.25×10 -5 mol / L, the molar mass ratio of chloroplatinic acid to sodium sulfite is 1:6, a pH regulator is added to make the pH of the reaction solution 8, and the reaction solution is allowed to stand at 30°C for 12 hours to obtain a platinum-cobalt precursor solution.
[0051] (2) Preparation of ultrafine platinum-cobalt alloy nanowires
[0052] The precursor concentration of preparing platinum-cobalt metal is 1.25×10 -5 mol / L aqueous solution, add vinylpyrrolidone and formic acid, make the precursor of platinum-cobalt metal, vinylpyrrolidone, and formic acid have a molar ratio of 1:100:40000 in turn, and move the obtained solution into a hydro...
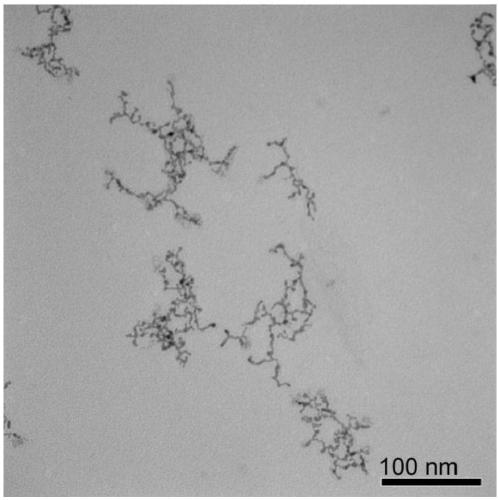
Embodiment 3
[0055] A water phase synthesis method of platinum-iron alloy nanowires, comprising the following steps:
[0056] (1) Preparation of platinum-iron precursor solution
[0057] Add chloroplatinic acid, ferric chloride, sodium sulfite, and pH regulator into water so that the concentration of chloroplatinic acid and ferric chloride is 1.25×10 -5 mol / L, the molar mass ratio of chloroplatinic acid to sodium sulfite is 1:6, adding a pH regulator to make the pH of the reaction solution 8, and standing at 30°C for 12 hours to obtain a platinum-iron precursor solution.
[0058] (2) Preparation of ultrafine platinum-iron alloy nanowires
[0059] The concentration of the precursor for preparing platinum-iron metal is 1.25×10 -5 mol / L aqueous solution, adding vinylpyrrolidone and formic acid, so that the molar ratio of the platinum-iron metal precursor, vinylpyrrolidone, and formic acid is 1:100:40000 in sequence, and the obtained solution is moved to a hydrothermal reaction kettle, and h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Average length | aaaaa | aaaaa |
| Average length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com