Boron carrying agent for integrating tumor diagnosis and treatment
A carrier agent and tumor technology, applied in the field of boron carrier agent for the integration of tumor diagnosis and treatment, can solve the problems of poor effect of BNCT therapeutic agents, high misdiagnosis rate, unstable diagnosis and treatment integration technology, etc., and achieve high early diagnosis accuracy, Pain-reducing, less toxic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、18
[0042] Embodiment 1, 18 Preparation of F-FBY
[0043] 1. Dissolve p-hydroxyphenylacetaldehyde (1eq) and R-tert-butylsulfinamide (1.2eq) in 100ml tetrahydrofuran, add tetraisopropyl titanate (2eq), stir overnight at room temperature, add water to quench the reaction, and filter After drying, the solvent was removed by rotary evaporation and separated by column chromatography to obtain the corresponding imine.
[0044] 2. The toluene solution of the imine (1eq) prepared in step 1 and the pinacol borate (2eq) was added in advance by tricyclohexylphosphine fluoroborate (1.2%eq), copper sulfate (1.2%eq) , In the catalyst prepared by benzylamine (5%eq), stir at room temperature for 24h.
[0045] 3. Add ethyl acetate to dilute the reaction product of step 2, add 4M hydrochloric acid and 3M potassium bifluoride solution, and add acetonitrile to dilute to a reaction system of acetonitrile: water = 1:1, stir at room temperature for 2 hours, and then remove the solvent by rotary evapor...
Embodiment 2、18
[0051] Embodiment 2, 18 PET images of F-FBY in tumor-bearing mice
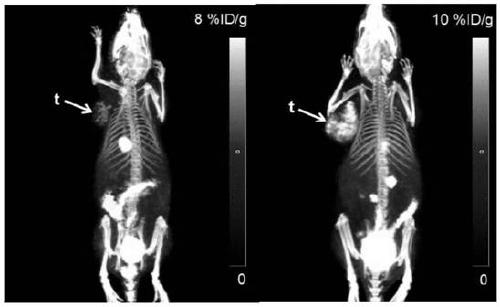
[0052] About 3.7MBq 18 F-FBY was injected into tumor-bearing mice through the tail vein under isoflurane anesthesia, and 10 minutes of static acquisition was performed 60 minutes after the injection to obtain PET image data. The result is as image 3 as shown, image 3 show 18 F-FBY is mainly metabolized by the kidney and bladder. Except for the gallbladder, kidney, bladder and other major metabolic organs, there is no obvious uptake in other normal tissues, and the uptake in the tumor site is obvious.
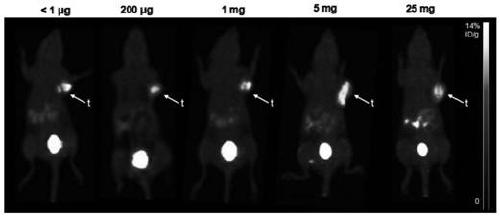
[0053] Embodiment 3, 18 PET images of F-FBY applied to tumor-bearing mice at different doses
[0054] Under isoflurane anesthesia, approximately 3.7 MBq 18 F-FBY mixed with different doses of FBY (0.2mg, 1mg, 5mg, 25mg) was injected into the tumor-bearing mice through the tail vein, and 10min of static acquisition was performed 90min after the injection to obtain PET image data. The result is as Figu...
Embodiment 4、18
[0056] Embodiment 4, 18 Distribution of F-FBY in different organs of tumor-bearing mice
[0057] About 3.7MBq 18 F-FBY mixed with 25 mg of FBY was injected into the tumor-bearing mice through the tail vein under isoflurane anesthesia, and the PET image data was obtained by static acquisition for 10 min at 90 min after the injection ( Figure 5 ). Figure 5 The results indicated that FBY can be enriched in a large amount in the tumor site, which fully meets the boron content required by BNCT, and can be used as a potential boron carrier in BNCT.
[0058] Immediately after the static acquisition of PET image data scanning, the mice were sacrificed, and different organs (brain, muscle, tumor, etc.) were digested by microwave and the boron concentration was measured by ICP-OES ( Figure 6 ). Figure 6 The results showed that at different FBY doses, the boron content of each organ determined by ICP-OES had a good correspondence with the radioactive signal, indicating that the F...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com