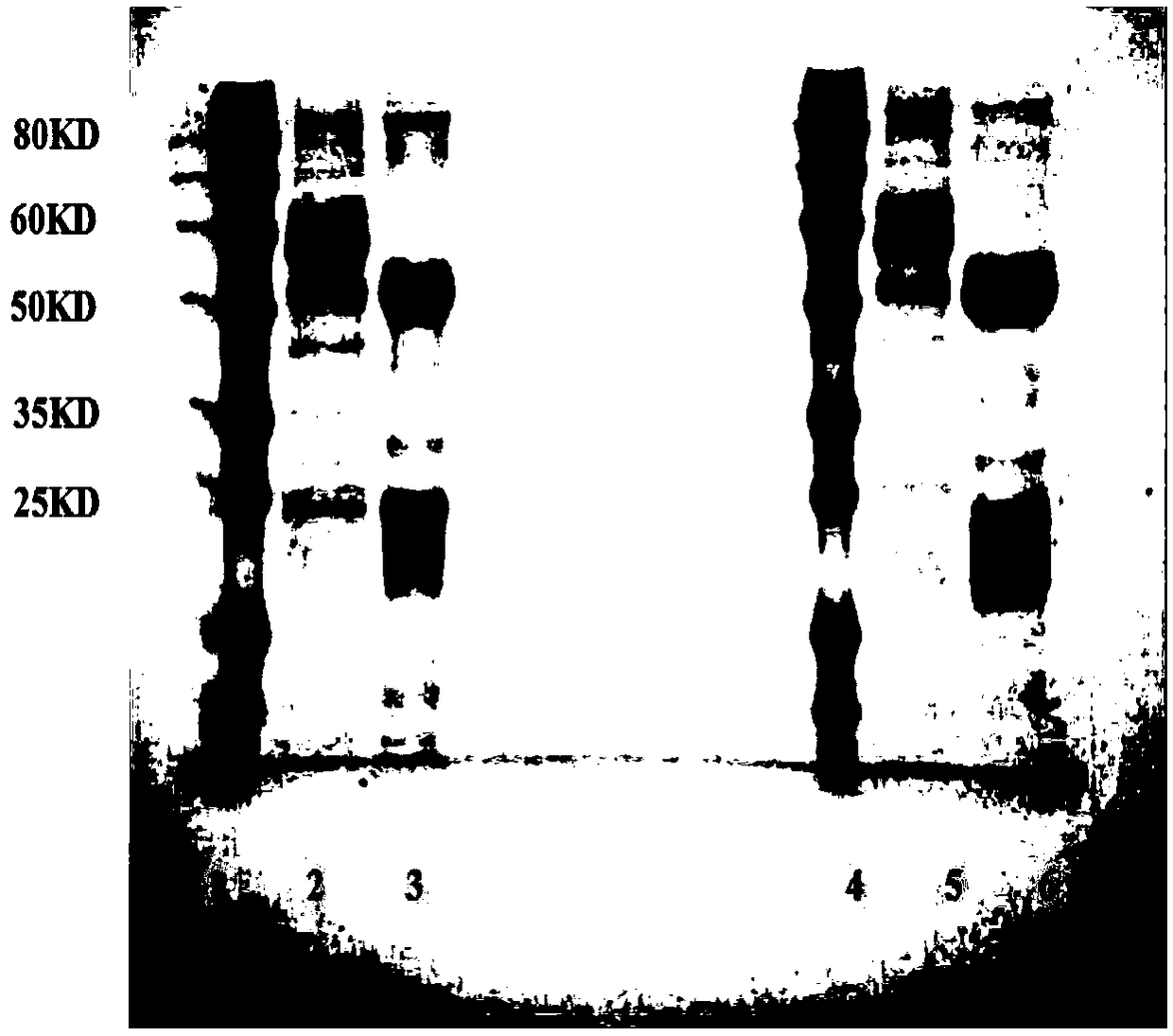
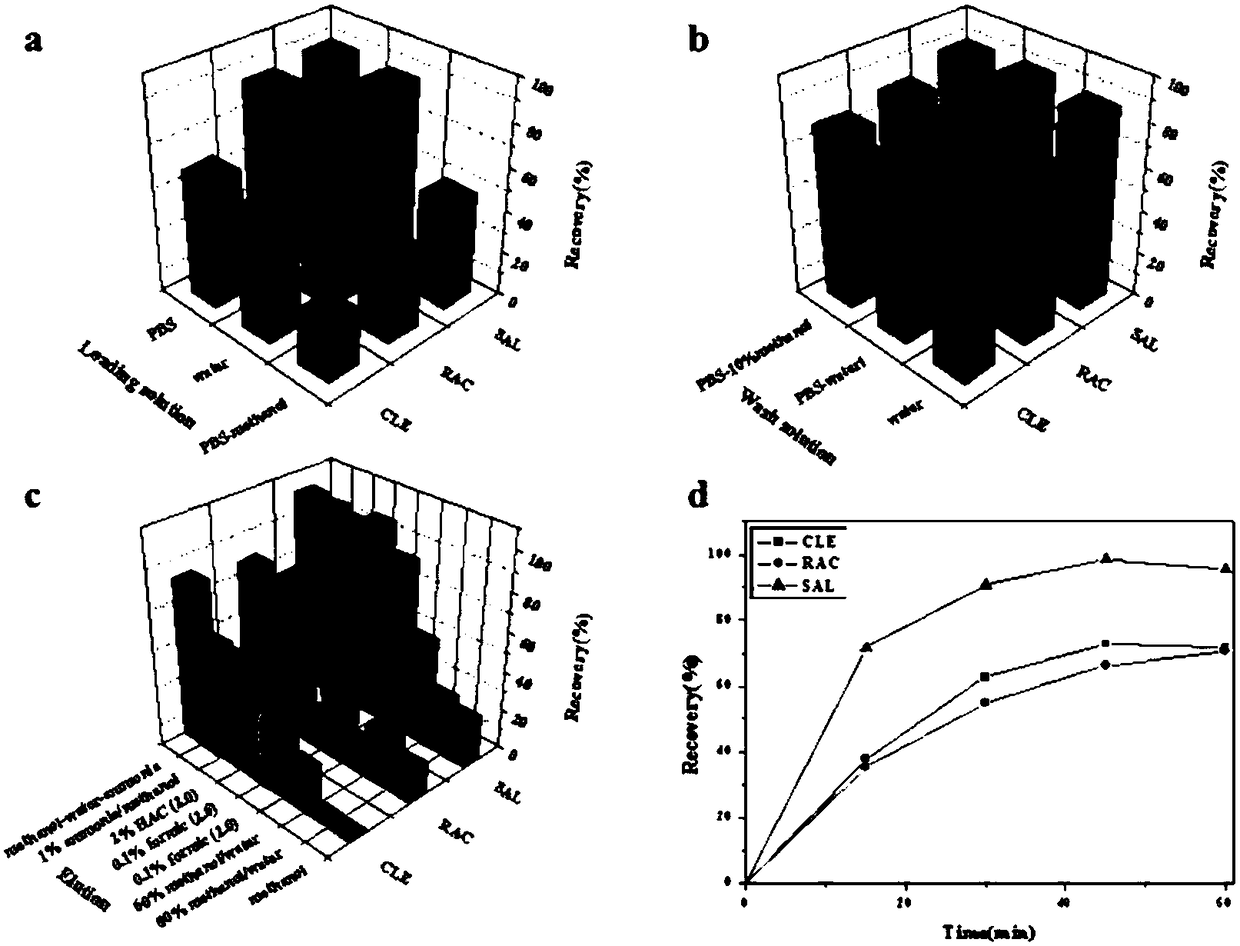
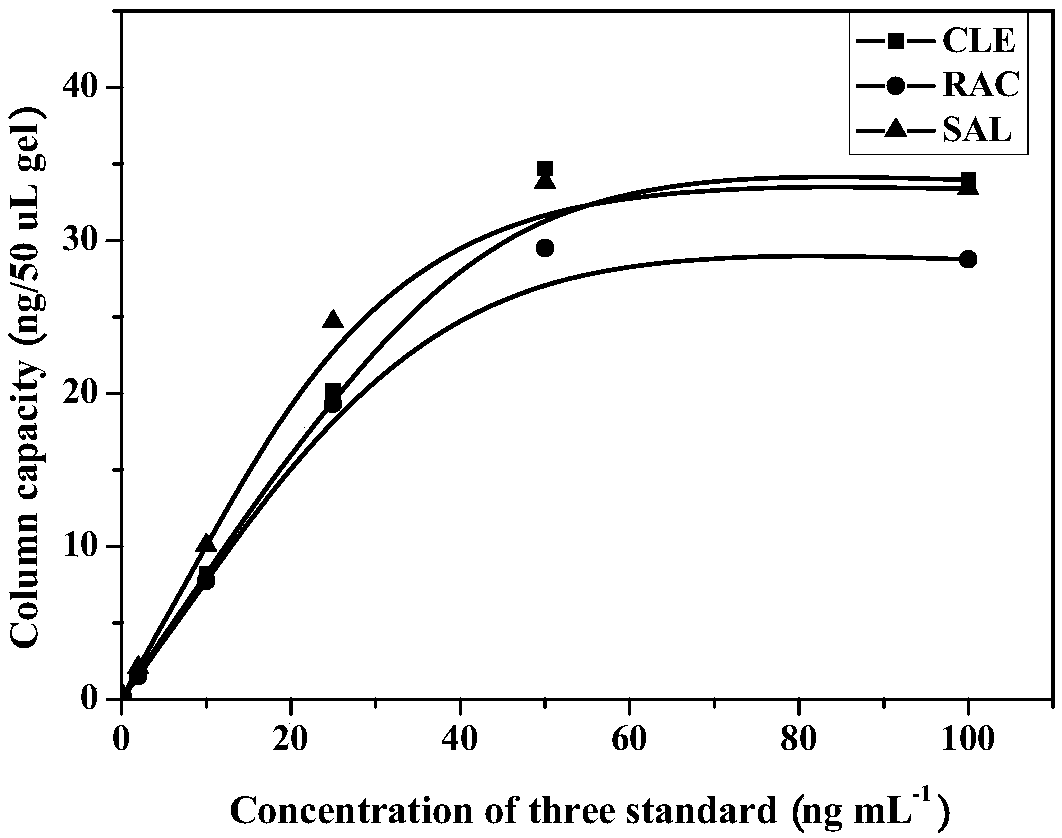
Anti-beta receptor agonist cluster-specific monoclonal antibody hybridoma cell line, monoclonal antibody secreted by cell line, and applications of monoclonal antibody
A hybridoma cell line and receptor agonist technology, applied in the biological field, can solve the problems of unavoidable mixing of matrix interfering substances, difficulty, and many operation steps, and achieve the effect of the best standard addition recovery rate and purification processing capacity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1: Acquisition of ascites samples containing monoclonal antibodies according to the present invention
[0071] 1. Recovery and proliferation of hybridoma cell line 1C3A and patent CN104311438A hybridoma cells
[0072] Take the cryopreservation tube of the hybridoma cell line from the liquid nitrogen tank, thaw it quickly in a water bath at 37°C, centrifuge at 600r / min for 5min, discard the supernatant, add 15% FBS / RPMI-1640 culture medium to suspend the cells, and add the above After the culture solution reaches 5ml, plant it in a 50ml cell culture bottle and culture it in a carbon dioxide incubator. After the cells grow to 30% density, change the medium halfway. - Passage once every 3 days according to 1:3-4.
[0073] 2. In vivo induction of monoclonal antibody protein and ascites acquisition
[0074] 7-10 days before planting hybridoma cells in vivo, 12 male BALB / c mice aged 8-10 weeks were injected intraperitoneally with 0.5ml / mouse of pristane, and careful...
Embodiment 2
[0077] Example 2: Purification of monoclonal antibody protein of the present invention and ractopamine monoclonal antibody of patent CN104311438A hybridoma cells
[0078] Aspirate 2mL protein G resin suspension, transfer it to a disposable PE column with a column capacity of 12mL, then add 20mL binding buffer (3.3768g Na 2 HPO 4 12H 2 O, 0.0888g NaH 2 PO 4 2H 2 O, 8.5g NaCl, 1LH 2 O preparation), the flow rate is 1mL min -1 , to remove impurities in the suspension and to precipitate the suspension. Then slowly add 4mL of monoclonal antibody ascites to make it slowly flow through the gel layer. Protein G is the cell wall protein of group G streptococci, which can capture the Fc region of IgG antibodies, while other subtypes cannot be captured, so as to achieve the purpose of purification . After the ascites has completely flowed through the gel layer, add 100 mL of binding buffer to wash to remove non-specific adsorption, and then add 15 mL of glycine buffer (0.1 mol L ...
Embodiment 3
[0080] Example 3: Analysis of Immune Crossover
[0081] The present invention selects clenbuterol hydrochloride, terbutaline, bromobuterol, sibuterol, and these four beta-adrenoceptor agonists to detect the cross-reaction rate. Albuterol concentration range 0-30ng mL -1 , the concentration range of cross-products is 0.1-10000ng mL -1 . Make standard curves for albuterol and other 4 cross-products, and calculate their respective ICs 50 , so as to obtain their respective CR values, and the results are shown in Table 1.
[0082] Table 1
[0083]
[0084] As can be seen from Table 1, when the CR value of albuterol was determined to be 100%, it was found that the cross-reaction rates of clenbuterol hydrochloride, terbutaline, bromobuterol and sibuterol were very large, and the CR values were respectively 66.8%, 57.3%, 42.1%, 121.5%. These four substances are very similar to salbutamol in structure, and all have a tert-butyl group connected to a nitrogen atom, indicating ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com