Preparation and Application of Tetraphenylpyrazine-Based Nitrile Vinyl Functionalized AIE Molecules
A technology of tetraphenylpyrazine and nitrile vinyl, which is applied in the preparation and application of AIE molecules, can solve the problems of easy photoisomerization and photooxidation, comprehension of complex AIE mechanism, poor chemical stability, etc., and achieve convenient Visual observation, fast response time, high sensitivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Synthesis of monoaldehyde substituted tetraphenylpyrazine derivatives:
[0038]
[0039] Add 3g (6.5mmol) monobromosubstituted tetraphenylpyrazine (refer to J.Mater.Chem.C, 2016, 4, 2901 for the synthetic route), 1.17g (7.8mmol) p-formylphenylboronic acid in a 250mL round bottom flask and 372 mg (0.32 mmol) tetrakistriphenylphosphine palladium. After the system was evacuated and nitrogen exchanged three times, 100 mL of distilled tetrahydrofuran and 50 mL of 2M potassium carbonate aqueous solution were injected. The reaction system was stirred overnight at reflux. Subsequently, the system was returned to room temperature, extracted with dichloromethane and washed with water several times. The collected organic phase was distilled off under reduced pressure to remove the solvent. The crude product was purified by column chromatography with n-hexane / dichloromethane (volume ratio 1:1) to obtain 2.79 g (5.7 mmol) of a white solid with a yield of 88.2%. 1 H NMR (400MH...
Embodiment 2
[0043] Example 2 Time Response of Fluorescent Molecules to Hydrogen Sulfide
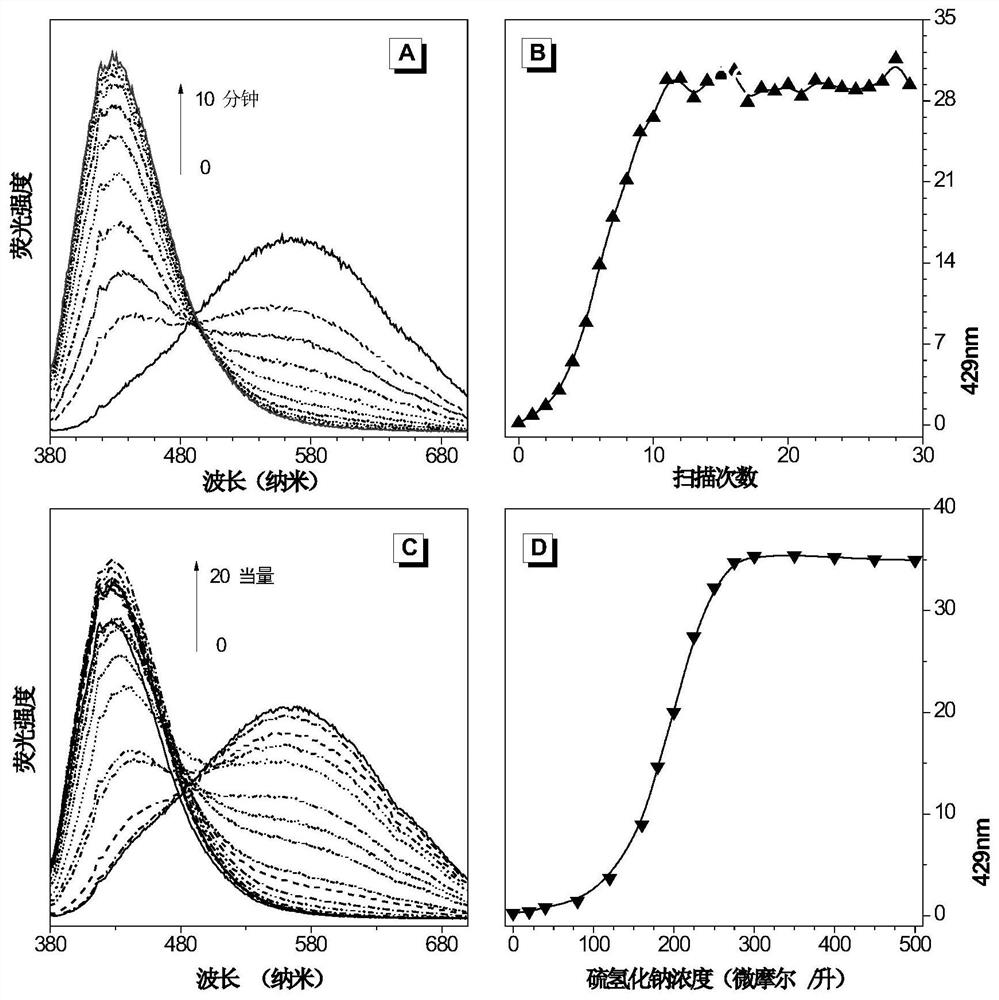
[0044] Fluorescent molecules were dissolved in dimethyl sulfoxide, and a stock solution with a concentration of 250 μM was prepared. Take 1mL of the mother liquor in a glass bottle, add 8mL of dimethyl sulfoxide, and then add 1mL of sodium hydrosulfide in PBS buffer (buffer concentration is 10mM, pH is 7.4), to ensure that the equivalent ratio of probe molecules to sodium hydrosulfide is 1 :10. Measure the fluorescence emission spectrum as a function of time with a fluorescence spectrometer. from figure 1 In (A) and (B), it can be seen that before adding sodium hydrosulfide solution, the probe emits orange light at 565nm; after adding sodium hydrogensulfide solution, as time goes on, the orange light gradually weakens, and the dark blue at 429nm The light was gradually increased, and the probe response was completed within 10 minutes.
Embodiment 3
[0045] Embodiment 3 Fluorescent molecules respond to the concentration of hydrogen sulfide
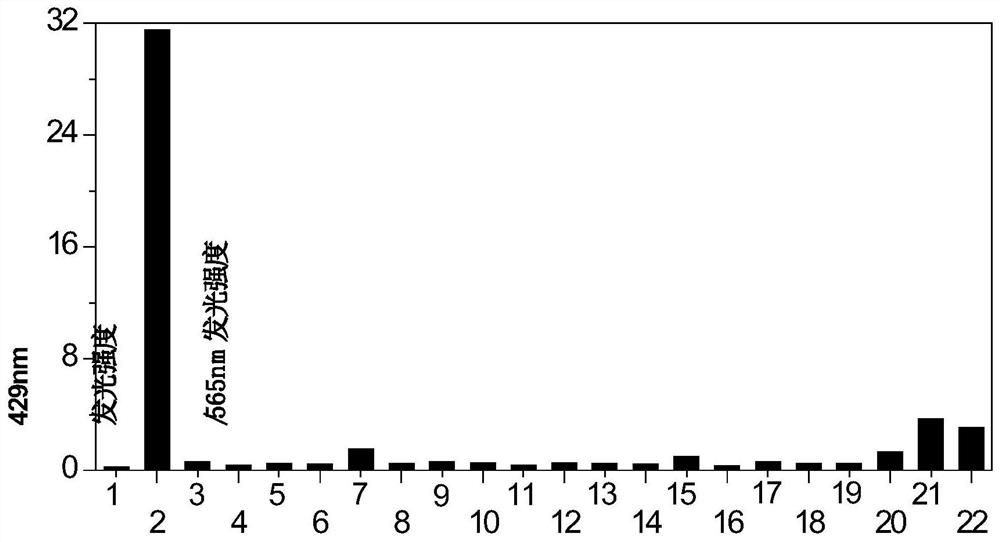
[0046] Fluorescent molecules were dissolved in dimethyl sulfoxide, and a stock solution with a concentration of 250 μM was prepared. Take 1mL of the mother liquor in a glass bottle, add 8mL of dimethyl sulfoxide, and then add 1mL of sodium hydrosulfide in PBS buffer (buffer concentration is 10mM, pH is 7.4), to ensure that the concentration of sodium hydrosulfide solution is in the range of 0-500μM Variety. Ten minutes after adding the sodium hydrosulfide solution, the fluorescence emission spectra were measured respectively. from figure 1 In (C) and (D), it can be seen that as the concentration of sodium hydrosulfide solution increases, the orange-yellow light emitted by the probe at 565nm gradually weakens, while the dark blue light at 429nm gradually increases, and the response ends at 250μM . Moreover, the ratio of the intensity of deep blue light at 429nm to the intensity of o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com