Full suspension culture method of duck hepatitis virus
A technology of duck hepatitis virus and culture method, which is applied in the field of veterinary biological products, can solve the problems of no cell-derived duck hepatitis virus vaccine, decreased culture titer of duck hepatitis virus, failure to meet production requirements, etc., to achieve large-scale cultivation, The effect of simplifying the product separation and purification process and improving the utilization rate of equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Effects of different doses of inoculation and culture time on the proliferation of duck hepatitis virus in passaged cells derived from duck embryo cells
[0032] The sources of materials used in Embodiment 1 of the present invention are as follows:
[0033] 1. Virus: duck hepatitis virus attenuated strain GD75, identified, kept and supplied by Zhaoqing Dahuanong Biological Pharmaceutical Co., Ltd.
[0034] 2. Cells: The whole suspension passage cell line is a passage cell line derived from duck embryo cells.
[0035] 3. Culture medium: the name is medium EBLM004, containing 10mg / L alanine, 10mg / L arginine, 10mg / L cysteine, 10mg / L tyrosine, 10mg / L chromosine amino acid, 10mg / L valine, 10mg / L leucine, 1mg / L vitamin C, 1mg / L biotin, 1mg / L folic acid, 1mg / L choline chloride, 1mg / L inositol, 1mg / L Niacinamide, 1mg / L Pyridoxine Hydrochloride, 50mg / L Potassium Chloride, 50mg / L Sodium Chloride, 50mg / L Disodium Hydrogen Phosphate, 50mg / L Sodium Bicarbonate, 1000mg / L...
Embodiment 2
[0046] Example 2: ELD of Duck Hepatitis Virus at Different Harvesting Time in Duck Embryo Cell-Derived Passage Cell Line Culture 50 Compare
[0047] The source of materials used in Embodiment 2 of the present invention is as follows:
[0048] 1. Virus: Adapted strain of duck hepatitis virus (GD75 strain) in passage cells derived from duck embryo cells.
[0049] 2. Cells: The whole suspension passage cell line is a passage cell line derived from duck embryo cells.
[0050] 3. Culture medium: the name is medium EBLM004, containing 10mg / L alanine, 10mg / L arginine, 10mg / L cysteine, 10mg / L tyrosine, 10mg / L chromosine amino acid, 10mg / L valine, 10mg / L leucine, 1mg / L vitamin C, 1mg / L biotin, 1mg / L folic acid, 1mg / L choline chloride, 1mg / L inositol, 1mg / L Niacinamide, 1mg / L Pyridoxine Hydrochloride, 50mg / L Potassium Chloride, 50mg / L Sodium Chloride, 50mg / L Disodium Hydrogen Phosphate, 50mg / L Sodium Bicarbonate, 1000mg / L Glucose, 0.1mg / L manganese sulfate, 0.01mg / L ferric nitrate,...
Embodiment 3
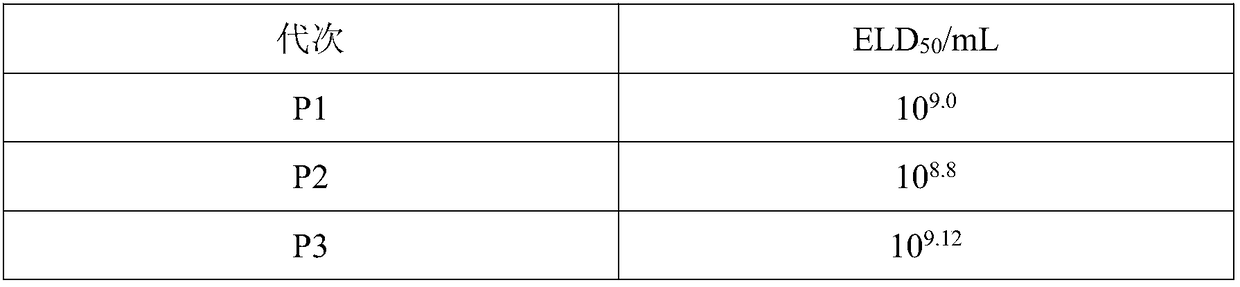
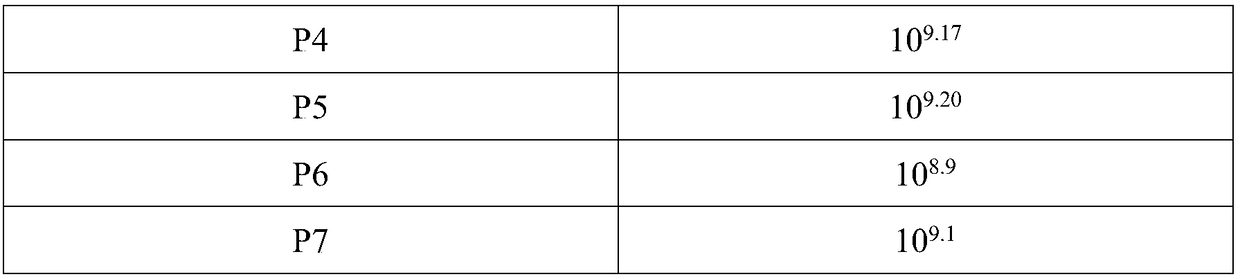
[0058] Example 3: Stable passage of duck hepatitis virus in passage cells derived from duck embryo cells
[0059] The present invention implements 3 sources of materials used as follows:
[0060] 1. Virus: ELD harvested from previous generation 50 The highest duck hepatitis virus was used as the seed virus.
[0061] 2. Cells: Passage cells derived from duck embryo cells of the whole suspension passage cell line.
[0062] 3. Culture medium: the name is medium EBLM004, containing 10mg / L alanine, 10mg / L arginine, 10mg / L cysteine, 10mg / L tyrosine, 10mg / L chromosine amino acid, 10mg / L valine, 10mg / L leucine, 1mg / L vitamin C, 1mg / L biotin, 1mg / L folic acid, 1mg / L choline chloride, 1mg / L inositol, 1mg / L Niacinamide, 1mg / L Pyridoxine Hydrochloride, 50mg / L Potassium Chloride, 50mg / L Sodium Chloride, 50mg / L Disodium Hydrogen Phosphate, 50mg / L Sodium Bicarbonate, 1000mg / L Glucose, 0.1mg / L manganese sulfate, 0.01mg / L ferric nitrate, 0.1mg / L insulin, 0.5mg / L EGF, 1mg / LbFGF.
[0063] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com