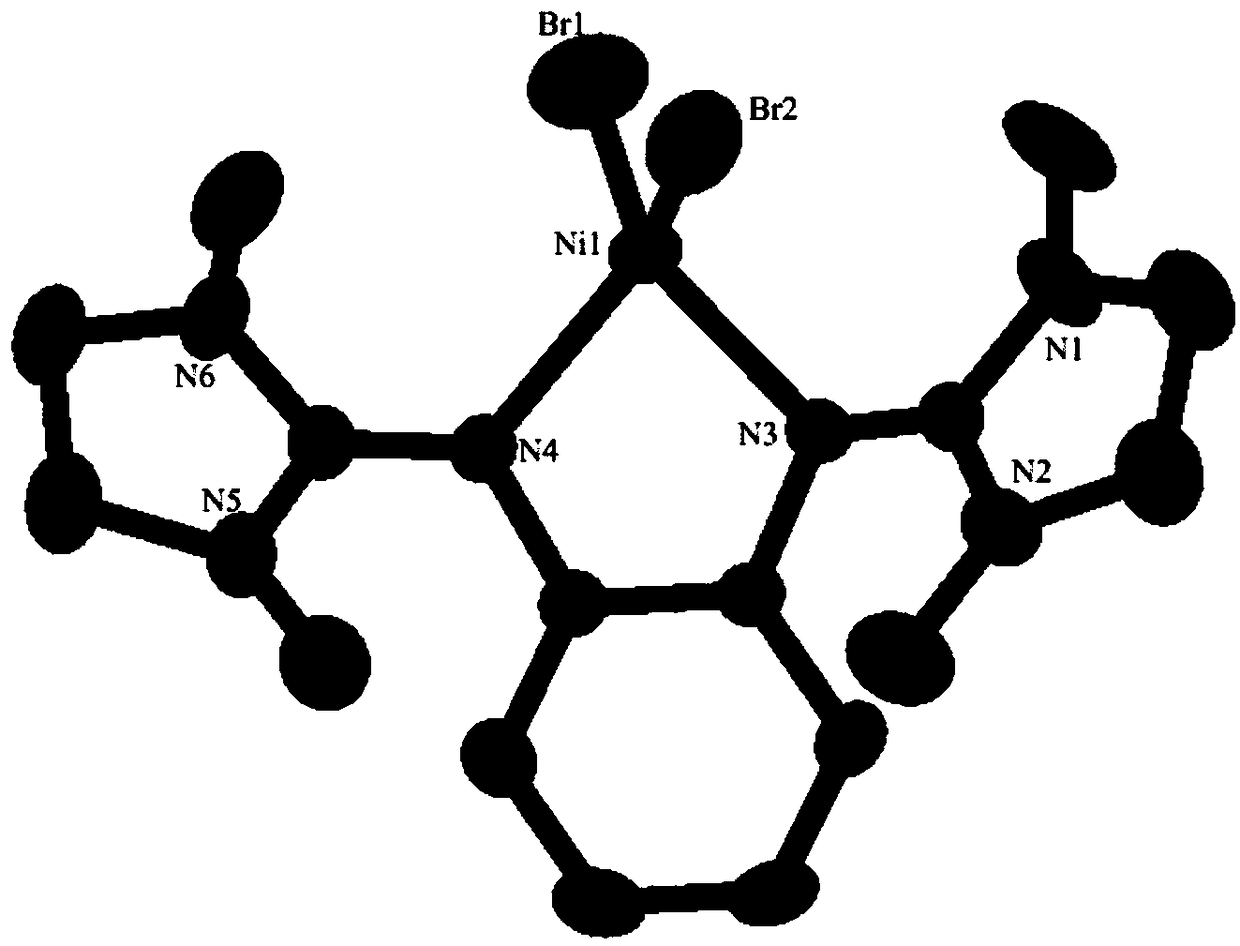
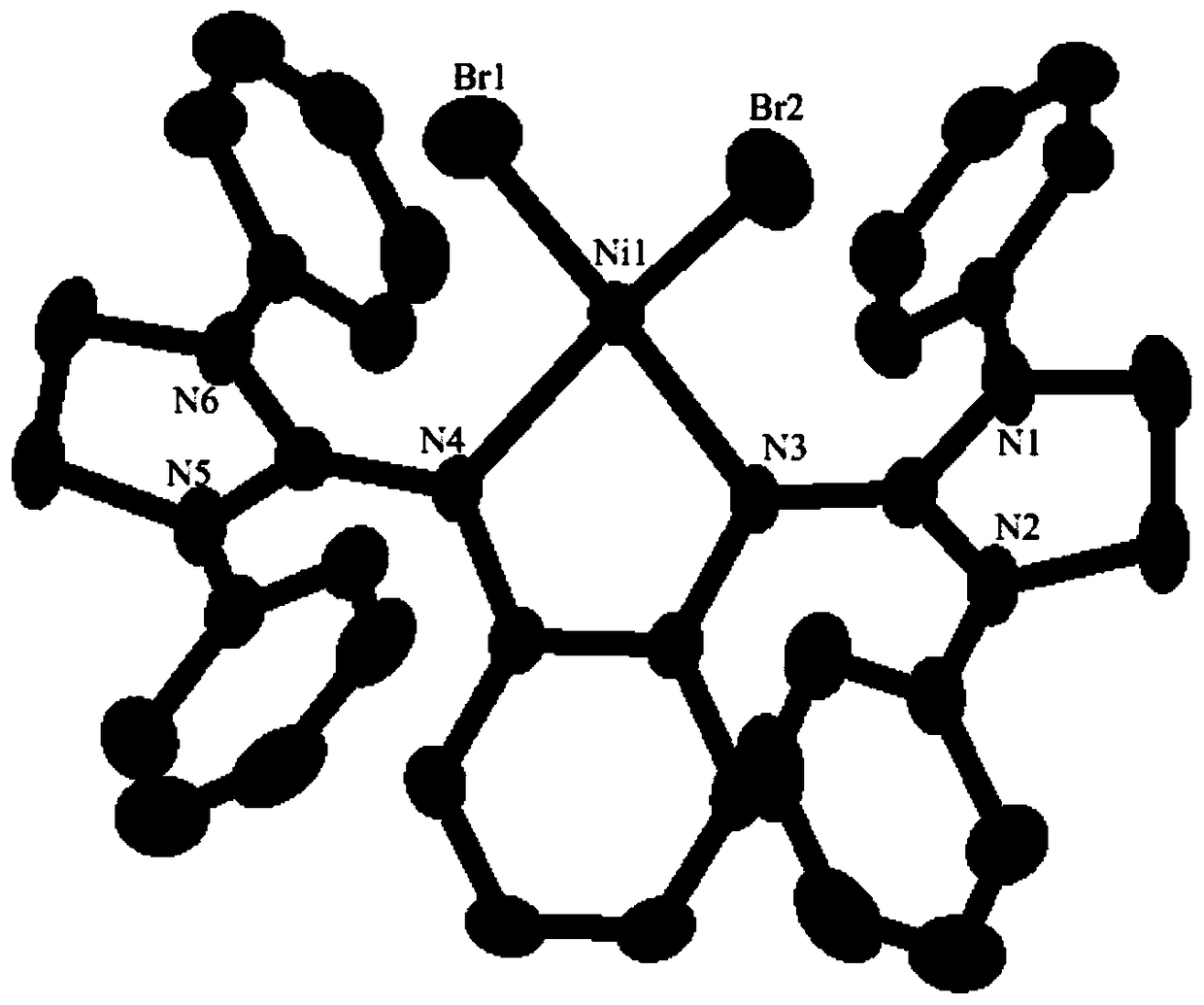
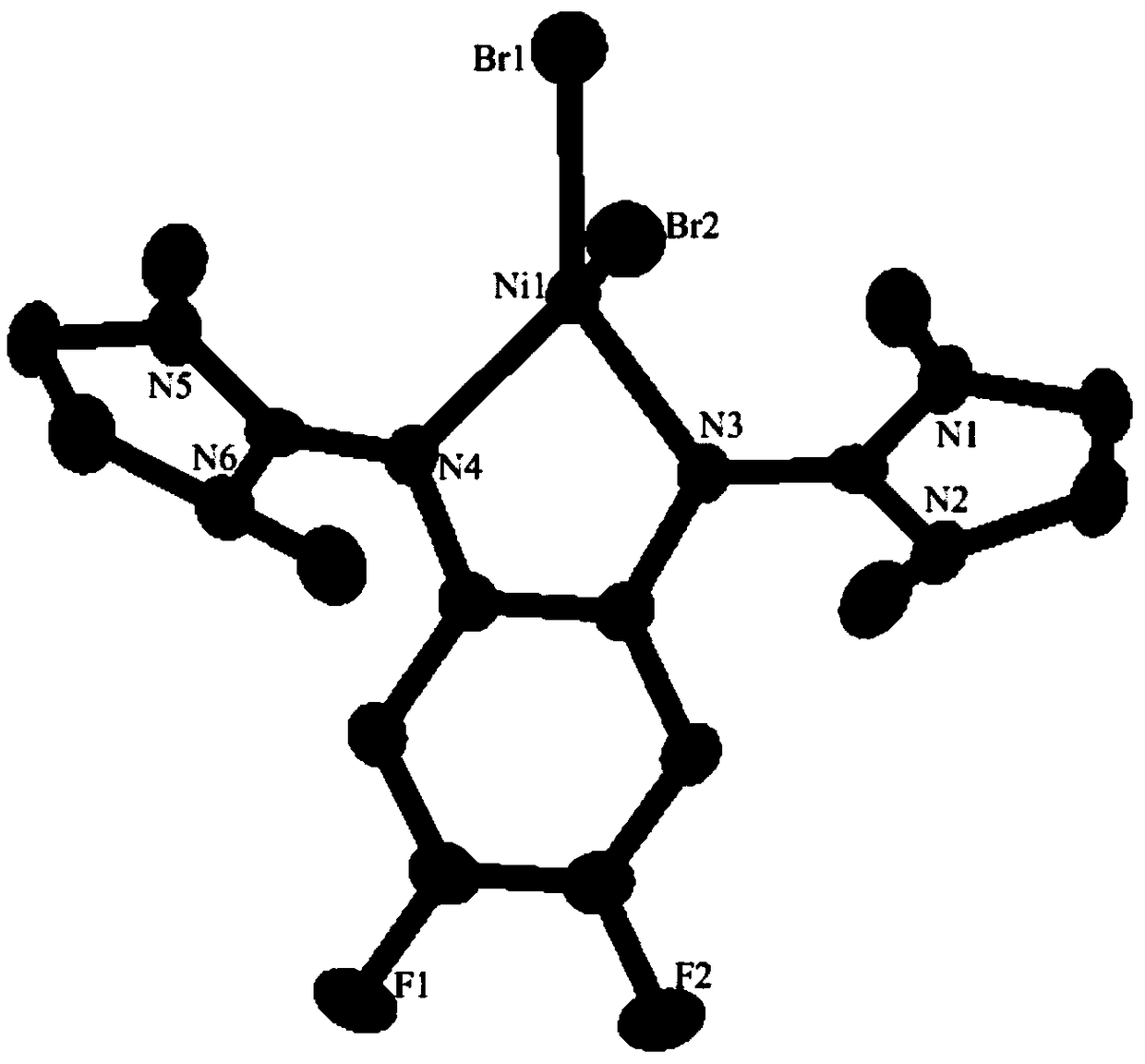
Ni-Pd metal complex as well as preparation method, application and product thereof and application of product
A technology of metal complexes and nickel-palladium, applied in the field of nickel-palladium metal complexes and their preparation, can solve the problems of uncontrollable regioselectivity and stereoregularity of polar monomers, nitrogen-heterocyclic carbene decomposition, and low activity , to achieve high catalytic activity and thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] The preparation method of metal complex, concrete steps are as follows:
[0085] (1) React urea compounds and oxalyl chloride in acetonitrile at 66°C for 12 hours to obtain imidazolidine chloride salt. After the reaction, the product is purified by recrystallization from toluene, wherein the chlorine atom in oxalyl chloride and urea The molar ratio of the compound is 5:1, and the urea compound is a compound with the structure of formula (a) and a compound with the structure of formula (c), in the formula, R 1 , R 4 , R 9 and R 12 is methyl, R 2 , R 3 , R 10 and R 11 is hydrogen, L is oxygen;
[0086] (2) react imidazolidine chloride salt, diamine compound and triethylamine in n-heptane at 60°C for 24h to obtain phenylenediamine imidazolidine ligand, wherein, imidazolidine chloride salt, diamine compound and The molar ratio of triethylamine is 2:1:4, and the structural formula of diamine compounds is as formula (e), wherein R 5 , R 6 , R 7 and R 8 is hydrogen...
Embodiment 2
[0091] The preparation method of metal complex, concrete steps are as follows:
[0092] (1) React urea compounds and phosgene in n-heptane at 60°C for 15 hours to obtain imidazolidine chloride salt. After the reaction, the product is purified by extraction with dichloromethane, wherein the chlorine atom in phosgene The molar ratio with urea compound is 4:1, and urea compound is the compound with formula (a) structure and the compound with formula (c) structure, in the formula, R 1 , R 4 , R 9 and R 12 is phenyl, R 2 , R 3 , R 10 and R 11 is hydrogen, L is oxygen;
[0093] (2) React imidazolidine chloride salts, diamine compounds and triethylamine in methylene chloride at 100°C for 10 h to obtain phenylenediamine imidazolidine ligands, wherein imidazolidine chloride salts, diamine compounds and The molar ratio of triethylamine is 2.3:1:2.2, and the structural formula of diamine compounds is as formula (e), wherein R 5 , R 6 , R 7 and R 8 is hydrogen;
[0094] (3) R...
Embodiment 3
[0098] The preparation method of metal complex, concrete steps are as follows:
[0099] (1) React urea compounds and triphosgene in toluene at 90° C. for 6 h to prepare imidazolidine chloride salts, wherein the molar ratio of chlorine atoms in triphosgene to urea compounds is 7:1, and urea compounds have The compound of formula (a) structure and the compound with formula (c) structure, in the formula, R 1 , R 4 , R 9 and R 12 is methyl, R 2 , R 3 , R 10 and R 11 is hydrogen, L is sulfur;
[0100] (2) React imidazolidine chloride salts, diamine compounds and triethylamine in acetonitrile at 90°C for 18 hours to obtain phenylenediamine imidazolidine ligands. After the reaction, the product is purified by extraction with chloroform , wherein, the molar ratio of imidazolidine chloride salt, diamine compound and triethylamine is 2:1:4, and the structural formula of diamine compound is as formula (e), wherein R 5 and R 8 for hydrogen, R 6 and R 7 is fluorine;
[0101] (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com