Preparation method of alectinib intermediate
An intermediate and tinib technology, which is applied in the field of preparation of alectinib intermediates, can solve the problems of poor product quality, expensive preparation costs of raw materials and reagents, and poor operational safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
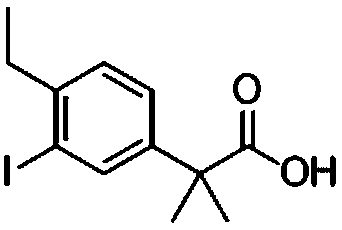
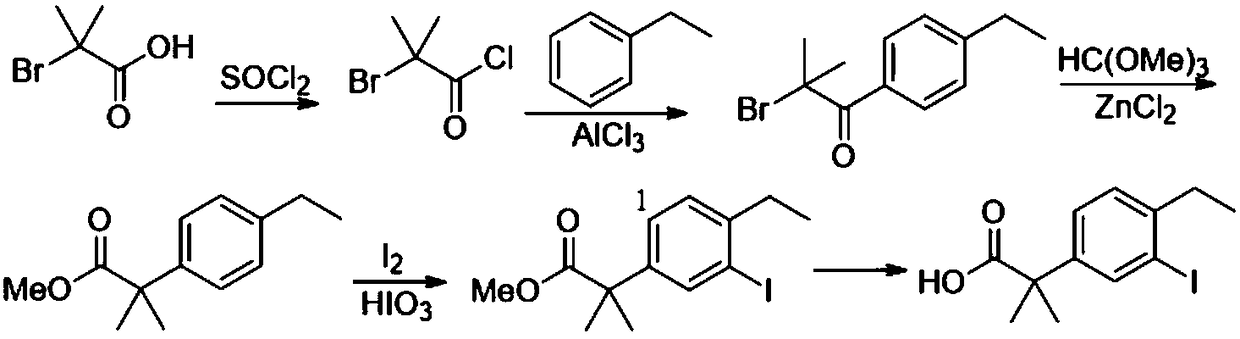
[0132] Embodiment 1, the preparation method of alectinib intermediate 2-(4-ethyl-3-iodo)phenyl-2-methylpropionic acid
[0133]1) Add 64.0g of diethyl malonate (compound A-2a) and 640mL of ethylene glycol dimethyl ether into the reaction flask, add 21.6g of sodium methoxide, heat up to 70-75°C for 0.5h, then add 7.6g of iodine Cuprous chloride and 5.8g cuprous bromide were stirred for 1h; 37.0g of bromoethylbenzene (compound A-1) was added and kept at 70-75°C for 10-12h. Add 50% sodium hydroxide aqueous solution, keep the pH>12, stir for 4 hours, filter, concentrate under reduced pressure to remove the solvent; add water and hydrochloric acid to adjust the pH to 1-2, stir, filter with suction, wash with water, dry, and recrystallize to obtain compound A- 3 (25.0 g, 76% yield).
[0134] 2) Add 20.0g of compound A-3, 100mL of methanol and 4mL of concentrated sulfuric acid into the reaction flask, heat up to reflux for 4-5h, concentrate under reduced pressure to remove methanol, ...
Embodiment 2
[0138] 1) Add 48.0g of diethyl malonate (compound A-2a) and 640mL of 1,4-dioxane into the reaction flask, add 12g of 60wt% sodium hydride, heat up to 70-75°C and react for 0.5h, Add 7.6g of cuprous iodide, add 37.0g of bromoethylbenzene (compound A-1) after stirring, and keep the reaction at 70-75°C for 10-12h. Add 50% sodium hydroxide aqueous solution, keep the pH > 12, stir for 4 hours, filter, concentrate under reduced pressure to remove the solvent; add water and hydrochloric acid to the residue to adjust the pH to 1-2, stir, filter with suction, wash with water, dry, and recrystallize to obtain Compound A-3 (24.7 g, yield 75%).
[0139] 2) Add 20.0g of compound A-3, 20mL of methanol and 3.5mL of concentrated sulfuric acid into the reaction flask, heat up to reflux for 4-5h, concentrate under reduced pressure to remove methanol, add water and ethyl acetate in turn, separate the layers, and combine the organic layers. After washing with saturated sodium bicarbonate, the or...
Embodiment 3
[0143] 1) Add 80.0g of diethyl malonate (compound A-2a) and 640mL of tetrahydrofuran into the reaction flask, add 56g of potassium tert-butoxide, heat up to 65-70°C and react for 0.5h, then add 17.2g of cuprous bromide, After stirring, 37.0 g of bromoethylbenzene (compound A-1) was added, and the reaction was maintained at 65-70° C. for 10-12 h. Then add 50% sodium hydroxide aqueous solution, keep the pH > 12 and stir for 4 hours, then filter, concentrate under reduced pressure to remove the solvent; add water and hydrochloric acid to the residue to adjust the pH to 1-2, after stirring, filter with suction, wash with water, dry, and weigh Crystallization gave compound A-3 (23.6 g, yield 72%).
[0144] 2) Add 20.0g of compound A-3, 200mL of methanol and 4mL of acetyl chloride into the reaction flask, raise the temperature to reflux for 4-5h, concentrate under reduced pressure to remove methanol, add water and ethyl acetate in sequence, separate the layers, combine the organic l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com