Method for testing aminoglycoside drug residue in animal derived food
An aminoglycoside and animal-derived technology, which is applied in the field of determination of aminoglycoside drug residues in animal-derived foods, can solve problems such as low sensitivity, limited detection categories of aminoglycoside antibiotics, and inability to meet actual monitoring requirements. Strong, simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Sample preparation
[0062] Take 500g of representative honey samples. For uncrystallized samples, stir vigorously evenly. For samples with crystallization, plug the cap of the sample bottle tightly and warm it in a water bath not exceeding 60°C. Stir evenly after the samples are completely melted. , rapidly cooled to room temperature. Care should be taken to prevent moisture evaporation during melting. The prepared samples were divided into two parts, put into sample bottles respectively, sealed, and marked with marks. One was used as a test sample, and the other was stored below -18°C.
[0063] Take 500g representative royal jelly sample, thaw it at room temperature, wait until the sample is completely melted, stir well, divide the sample into two parts, put them into sample bottles respectively, seal them, and mark them. One was used as a test sample, and the other was stored below -18°C.
[0064] During the operation of sample preparation, sample contamination o...
Embodiment 2
[0118] Embodiment 2 Linear Range and Linear Equation
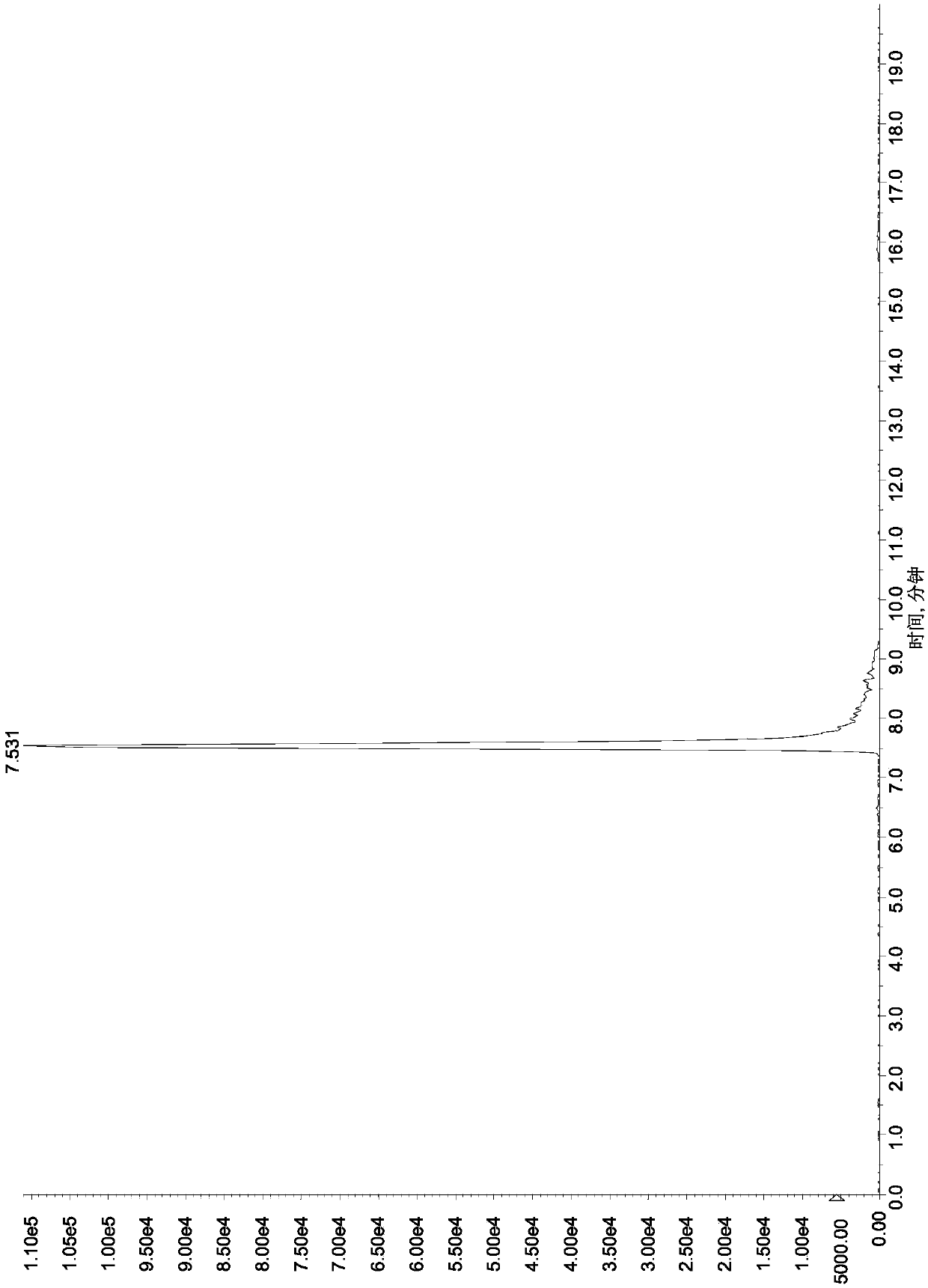
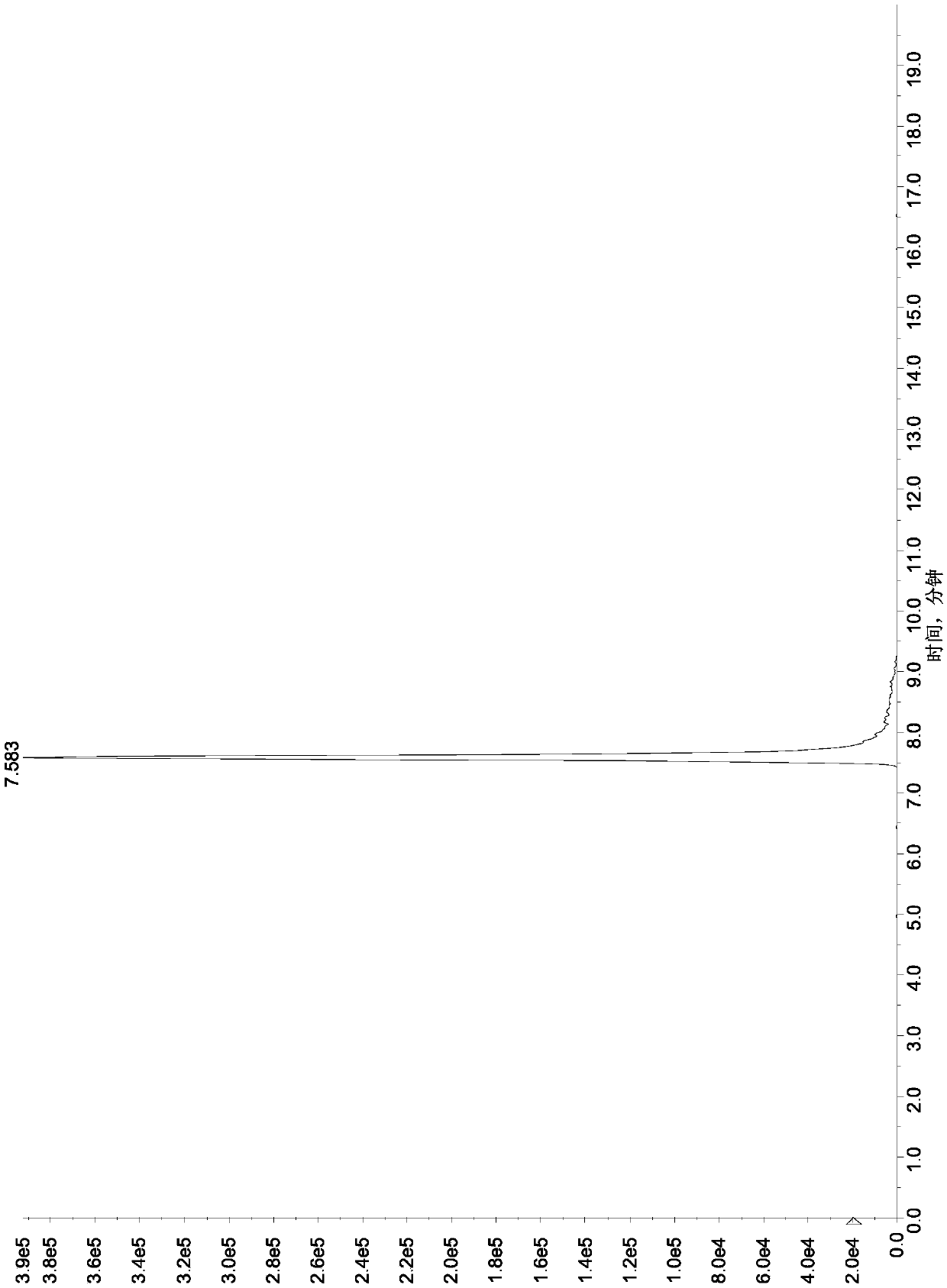
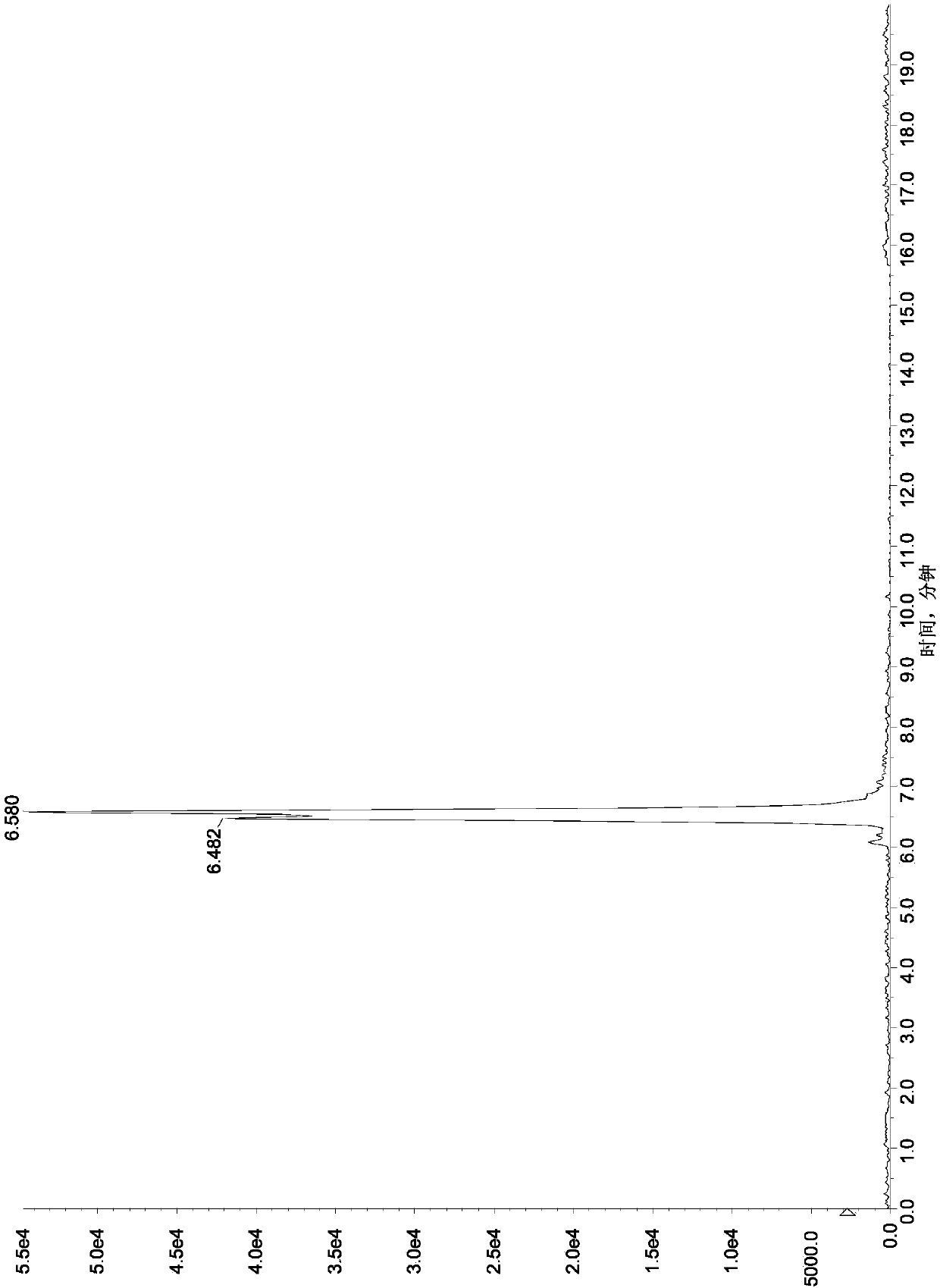
[0119]Referring to the method in Example 1, take a blank honey matrix solution, add a known amount of aminoglycosides, so that: streptomycin, dihydrostreptomycin, tobramycin, hygromycin B, ribomycin, western Somicin is 5ng / mL, 10ng / mL, 20ng / mL, 50ng / mL, 100ng / mL, spectinomycin, neomycin, apramycin, gentamicin, kanamycin, small promise 20ng / mL, 50ng / mL, 100ng / mL, 250ng / mL, 500ng / mL of Amikacin, Etimicin, Netilmicin, Paromomycin, Amikacin, respectively, as matrix matching mixed standard solution , carry out the liquid chromatography-mass spectrometry measurement, plot the corresponding mass concentration (X axis, ng / mL) with the response peak area (Y axis) of the quantitative ion, and the results are shown in Table 5. The results show that there is a good linear relationship between the response peak area of the quantitative ion and the sample concentration within the corresponding linear range of 16 kinds of aminoglycosi...
Embodiment 3
[0123] Embodiment 3 Determination of lower limit
[0124] Select a series of 16 aminoglycoside drug mixed matrix working standard solutions with lower mass concentrations, process and analyze and detect according to the method in Example 1 and chromatographic conditions, and calculate the lower limit of determination.
[0125] Measure and find that the method of the present invention is 5 μ g / kg to the assay low limit of streptomycin, dihydrostreptomycin, tobramycin, hygromycin B, ribomycin, sisomicin in honey; The lower limit of determination of amycin, neomycin, apramycin, gentamicin, kanamycin, micronomycin, paromomycin, amikacin, etimicin and netilmicin is 25 μg / kg. The lower limit of this method for determination of streptomycin, dihydrostreptomycin, tobramycin, hygromycin B, ribomycin and sisomicin in royal jelly is 10 μg / kg; spectinomycin, neomycin, The lower limit of determination of apramycin, gentamicin, kanamycin, micronomycin, paromomycin, amikacin, etilmicin and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com