Preparation method of solid polymer electrolyte and solid secondary battery thereof
A solid polymer and electrolyte technology, applied in secondary batteries, solid electrolytes, non-aqueous electrolytes, etc., can solve the problems of decreased recycling rate of electrode materials, high interface impedance, and inability to cope with the complex application environment of secondary batteries, and achieve Effect of improving dissociation rate and ion distribution uniformity, good stability and compatibility, and excellent cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] A method for preparing a polymer solid electrolyte, using the following steps:
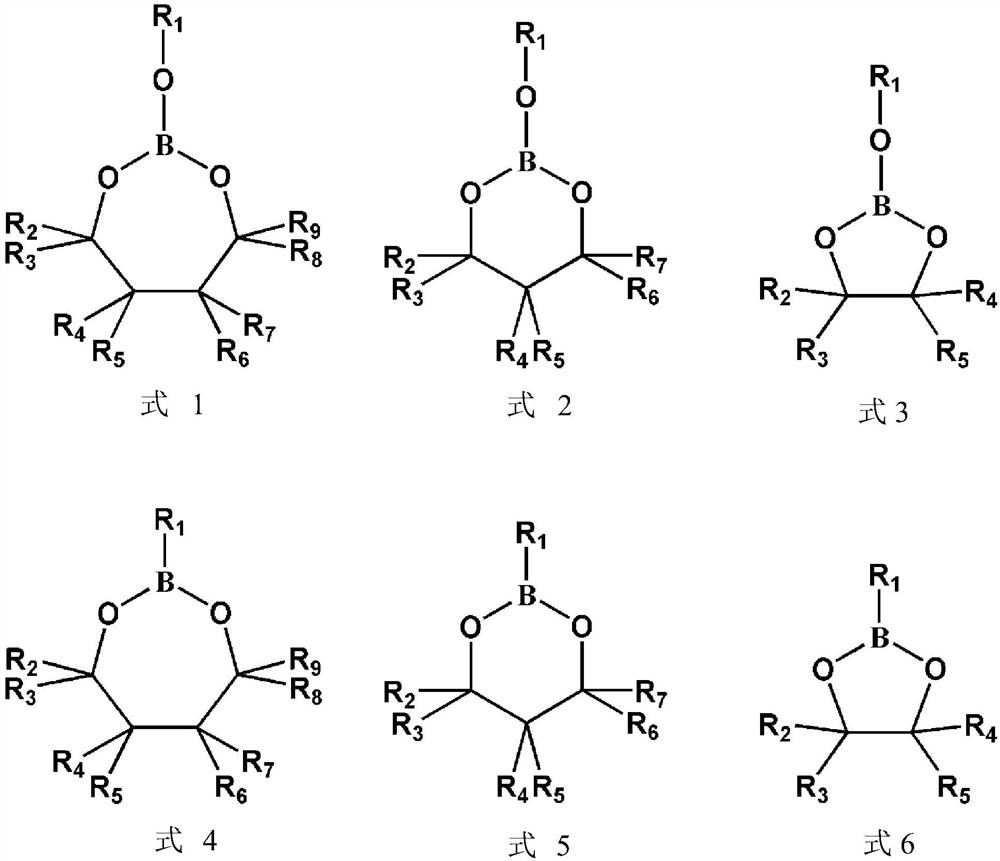
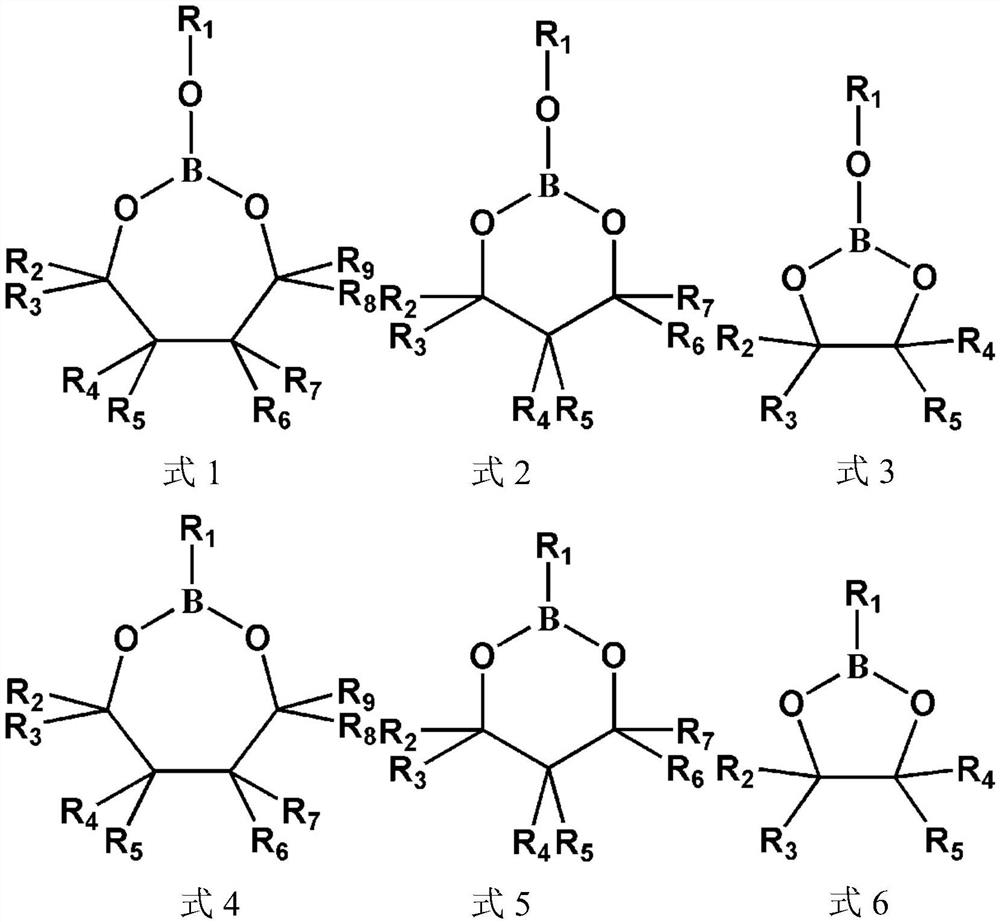
[0018] (I) In a glove box protected by argon and with water content and oxygen content less than 1ppm, mix 0.4g vinylene carbonate and 0.1g ethylenic boron-containing monomer compound After mixing with 0.1g lithium bistrifluoromethanesulfonylimide, add 0.0025mg of azobisisobutyronitrile as an initiator to prepare a precursor solution;
[0019] (II) In a glove box protected by argon and with water content and oxygen content less than 1ppm, inject the precursor solution prepared in step I into the porous cellulose membrane, and select two stainless steel sheets as blocking electrodes to assemble into a button-type battery, and then placed the battery at 60° C. for 24 hours for in-situ polymerization. The electrochemical workstation was used to carry out impedance spectroscopy test on the stainless steel sheet symmetrical battery assembled above, and the measured lithium ion conductivity was...
Embodiment 2
[0021] Select a metal lithium sheet as the electrode, and inject the precursor solution prepared in the first step of Example 1 into a porous cellulose membrane in a glove box protected by argon and with a water content and an oxygen content of less than 1 ppm to form a lithium metal symmetry battery, and heated at 60°C for 24h to in-situ polymerize the precursor to form a solid electrolyte. The steady-state current polarization test and the impedance spectrum test before and after polarization were carried out on the above-mentioned lithium metal symmetric battery by using an electrochemical workstation, and the measured lithium ion migration number was 0.68. As a comparison, a lithium metal symmetric battery assembled with an in-situ polymer solid-state electrolyte containing only vinylene carbonate but no ethylenic boron-containing monomer was tested, and its lithium ion migration number was only 0.43.
Embodiment 3
[0023] The positive electrode of lithium iron phosphate is selected, and the metal lithium sheet is used as the negative electrode, and the precursor solution prepared in the first step of Example 1 is injected into the porous cellulose membrane in a glove box protected by argon and with a water content and an oxygen content of less than 1ppm. Seal it, assemble it into a lithium iron phosphate / lithium metal battery, and heat it at 60°C for 24 hours to polymerize the precursor in situ to obtain a solid lithium iron phosphate / lithium metal battery. As a comparison, a liquid lithium iron phosphate / lithium metal battery was assembled with an organic electrolyte, and the two kinds of lithium iron phosphate / lithium metal batteries assembled above were subjected to a constant current charge and discharge test at a rate of 1C. The test voltage range was 2.5-4V. The initial discharge specific capacity of the solid lithium iron phosphate / lithium metal battery is 141.2mAh / g, which can be ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com