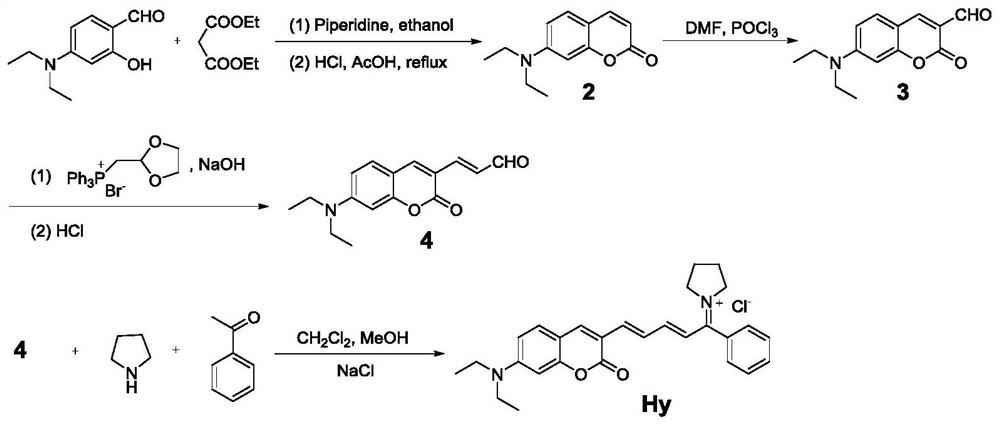
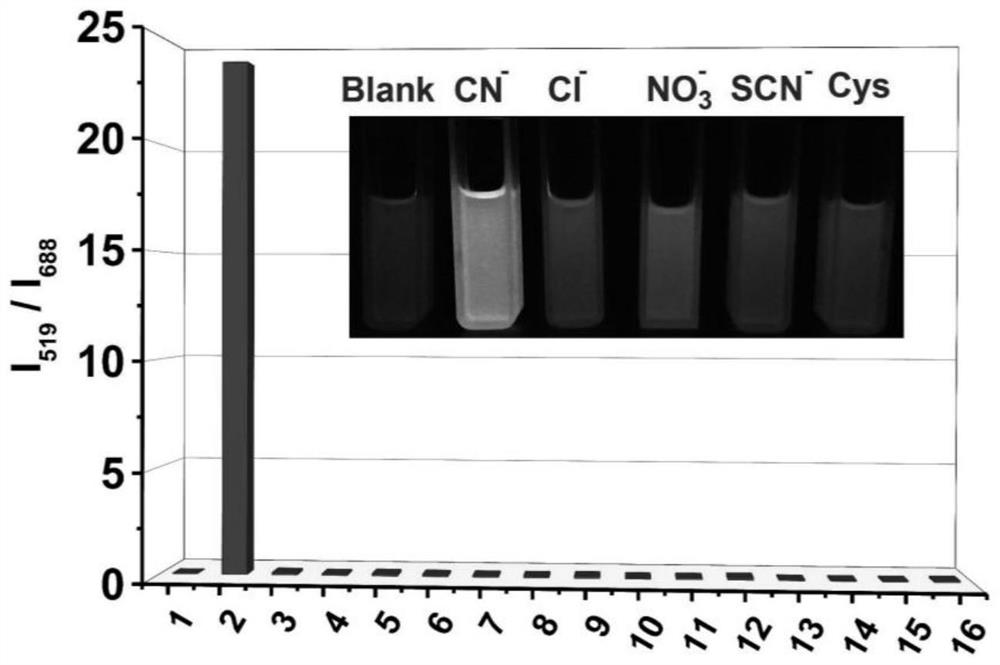
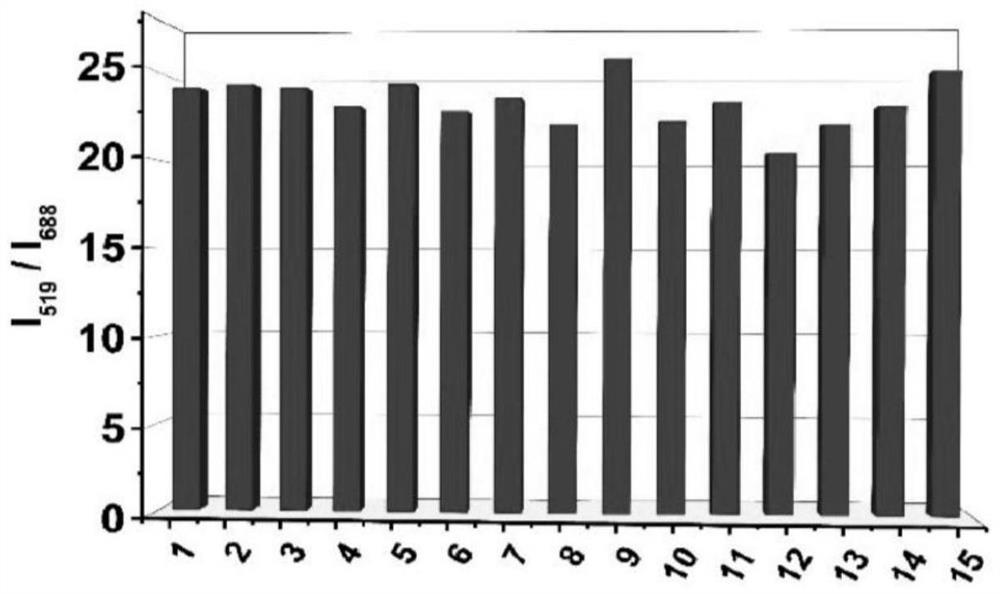
A preparation method and application of a ratiometric near-infrared fluorescent probe for detecting cyanide
A fluorescent probe and cyanide technology, which is applied in the field of fluorescence detection, can solve the problems of fluorescent signal artifacts and the reduction of detection accuracy of fluorescence analysis, and achieve the effects of reducing difficult-to-separate impurities, preventing side reactions and improving accuracy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) Add 3g of 4-(diethylamino) salicylaldehyde in a 150mL flask, dissolve it with 20mL of absolute ethanol, then add 4.7mL of diethyl malonate, stir well, then add 0.83mL of piperidine to obtain For mixed solution A, heat the mixed solution at 90°C to reflux and stir for 7 hours; after the reaction, cool to room temperature, use a rotary evaporator to remove excess solvent, and add 20 mL of the same amount of acetic acid and hydrochloric acid sequentially under ice-water bath conditions, followed by 100 The reaction was carried out under reflux at ℃ for 12 hours; after the reaction was completed, the pH was adjusted to 5 with sodium hydroxide solution in an ice-water bath, and the precipitate was filtered under reduced pressure to obtain a filter cake, which was then recrystallized with absolute ethanol at 90°C and dried in vacuo. Obtain 7-(diethylamino)coumarin as a khaki solid;
[0043] (2) First prepare the Wells reagent with 5mL of phosphorus oxychloride and an equa...
Embodiment 2
[0047] (1) Add 4g of 4-(diethylamino) salicylaldehyde to a 150mL flask, dissolve it in 25mL of absolute ethanol, then add 4mL of diethyl malonate, stir well, then add 0.5mL of piperidine to obtain a mixture Solution A: Heat the mixed solution at 90°C to reflux and stir for 7 hours; after the reaction, cool to room temperature, use a rotary evaporator to remove excess solvent, add 15mL of the same amount of acetic acid and hydrochloric acid successively under the condition of an ice-water bath, and then reflux at 105°C Heat to reflux for reaction for 12 hours; after the reaction, adjust the pH to 5 with sodium hydroxide solution in an ice-water bath, filter the precipitate under reduced pressure to obtain a filter cake, recrystallize with absolute ethanol at 90°C, and dry in vacuo to obtain 7-(diethylamino)coumarin khaki solid;
[0048] (2) First prepare the Wells reagent with 5mL of phosphorus oxychloride and an equal volume of DMF, then use 3.5mL of DMF to completely dissolve...
Embodiment 3
[0052] (1) Add 3g of 4-(diethylamino) salicylaldehyde to a 150mL flask, dissolve it with 30mL of absolute ethanol, then add 6mL of diethyl malonate, stir well, then add 1mL of piperidine to obtain a mixed solution A. Heat the mixture at 95°C to reflux and stir for 6 hours; after the reaction, cool to room temperature, use a rotary evaporator to remove excess solvent, add 10mL of acetic acid and hydrochloric acid in the same amount in an ice-water bath, and then heat at 100°C The reaction was carried out under reflux for 13 hours; after the reaction was completed, the pH was adjusted to 5 with sodium hydroxide solution in an ice-water bath, and the precipitate was filtered under reduced pressure to obtain a filter cake, which was then recrystallized with absolute ethanol at 90°C and dried in vacuo to obtain 7 -(diethylamino)coumarin khaki solid;
[0053] (2) First prepare the Wells reagent with 5mL of phosphorus oxychloride and an equal volume of DMF, then completely dissolve t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com