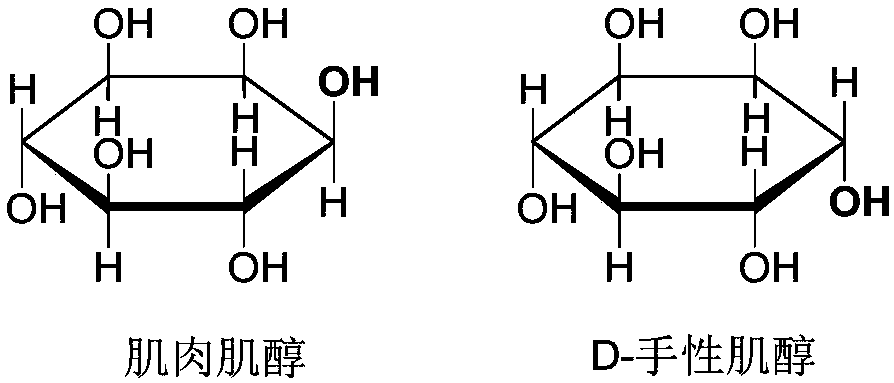
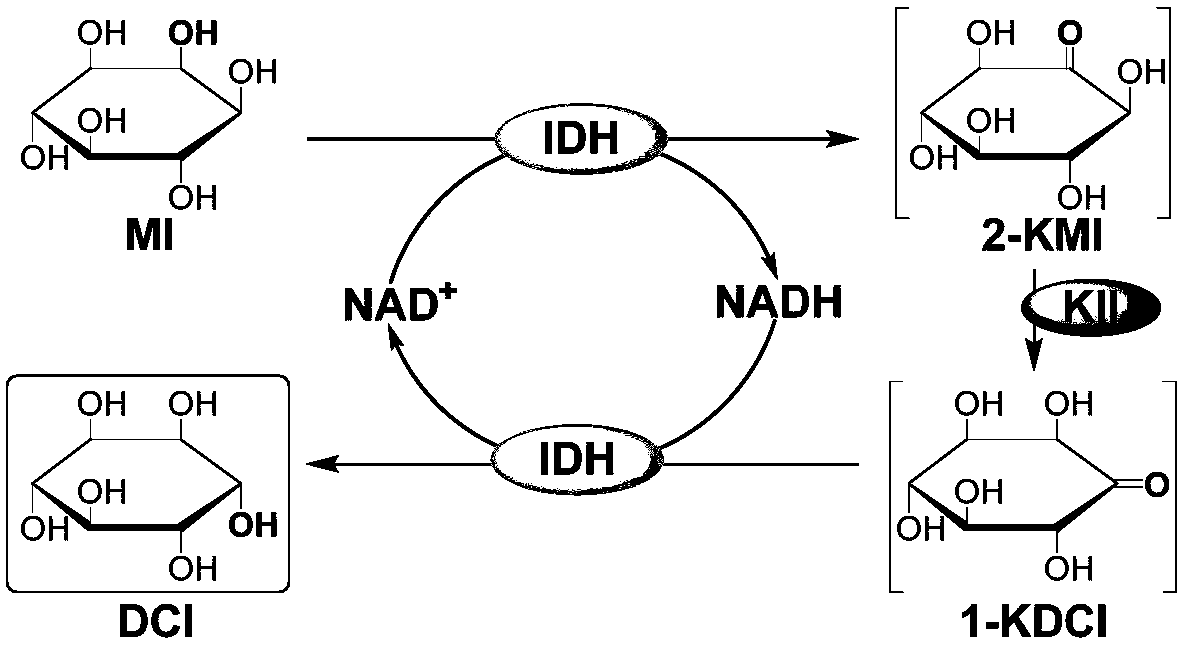
Preparation method of D-chiro-inositol
A technology of chiral inositol and myositol, applied in the field of bioengineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] The cloning of embodiment 1iolG and iolI gene
[0058]In this example, the thermostable myo-inositol dehydrogenase is from Geobacillus kaustophilus, and the NCBI accession number is WP_011231389; the thermostable inositol monoketone isomerase is also from Geobacillus kaustophilus, and the NCBI accession number is WP_042380806. Geobacillus kaustophilus was purchased from China General Microorganism Culture Collection Center (CGMCC). These two genes were obtained from genomic DNA by PCR using different primers: Geobacillus kaustophilus was inoculated in 5 mL liquid nutrient gravy agar medium (peptone 10 g / L, beef extract 3 g / L, NaCl 5 g / L, adjusted to pH 7.0), cultured at 55°C until the logarithmic growth phase, and the genome was extracted using DNA Kit (Tiangen Biochemical Technology Co., Ltd., China). Use primers iolG-F (containing BamHI restriction site): 5′-CGCGGATCCGATGACTCGGGTGAAAGTAGG-3′ and iolG-R (containing SalI restriction site): 5′-ACGCGTCGACTTATTTTGACGGAGCT...
Embodiment 2
[0060] The construction of embodiment 2 recombinant engineering bacteria
[0061] (1) Construction of pETDuet-iolG
[0062] Digest the pETDuet-1 plasmid and the iolG gene containing two restriction sites obtained by PCR in Example 1 with BamHI / SalI double enzymes at 37°C, and recover the double-digested target fragment and expression vector respectively , which was ligated at 22° C. for 5 h under the action of T4 ligase. The ligation product was transformed into competent E.coli Top10 by the calcium chloride method, and the transformants were selected for colony PCR and double enzyme digestion identification, and 2-3 positive transformants were selected for further verification by sequencing. The sequencing results showed that pETDuet-iolG recombination was successfully obtained carrier.
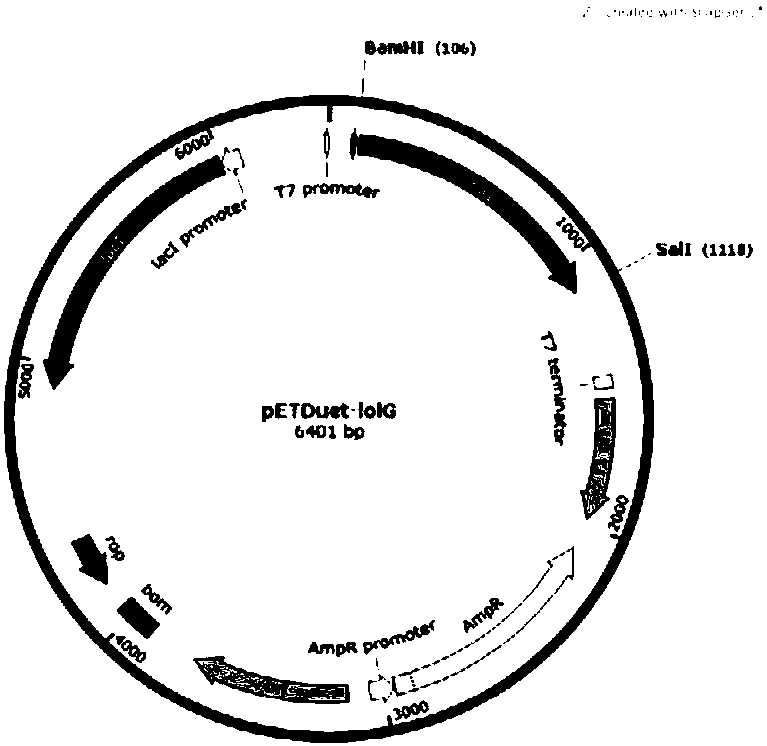
[0063] The plasmid map of pETDuet-iolG is as follows Figure 3A shown.
[0064] (2) Construction of pET29a-iolI
[0065] Use NdeI / KpnI double enzymes to respectively digest the pET-29a(...
Embodiment 3
[0072] The preparation of the whole cell of embodiment 3 recombinant engineering bacteria
[0073] Recombinant engineered bacteria BL21(DE3) / pETDuet-iolG, BL21(DE3) / pET29a-iolI and BL21(DE3) / pETDuet-iolG-iolI were picked and inoculated in LB medium containing ampicillin, cultured overnight at 37°C with shaking . The culture was transferred to fresh LB medium containing ampicillin at 1% inoculum size, and cultured with shaking at 37°C until OD 600 =0.6-0.8, use the final concentration of 0.1mM IPTG to induce overnight at 18°C, centrifuge at 5500rpm for 10min, discard the supernatant, and obtain whole cells expressing thermostable myo-inositol dehydrogenase, expressing thermostable myo-inositol monoketone isomer Enzymes as well as whole cells co-expressing thermostable myo-inositol dehydrogenase and thermostable myo-inositol monoketone isomerase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com