Nylon engineering plastic high-valued electrode active material and preparation method thereof
A technology of electrode active materials and engineering plastics, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of expensive conjugated organic electrode materials, gaps in specific capacity and rate performance, and restrictions on wide application, and achieve benefits that can be achieved The effect of continuous development, increased added value, and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In this embodiment one, pure nylon 6 is used as the electrode active material, specifically:
[0029] Mix and grind commercially available nylon 6, conductive agent (SP) and polyvinylidene fluoride (PVDF) evenly according to the mass ratio of 6:3:1, add an appropriate amount of N-methyl-2-pyrrolidone (NMP) dropwise according to the situation, and grind Stir to form a stable slurry. Coat it into a 0.06mm-thick pole piece, dry it in a blast drying oven at 60°C for 3-6 hours, then move it into a vacuum drying oven and dry it at 80°C for 12 hours, take it out after cooling, and obtain the working electrode. The prepared working electrode was cut into pole pieces with a diameter of 10mm, and half-cell assembly was carried out in a glove box, wherein the electrolyte was 1M LiPF 6 , the electrolyte is prepared by mixing ethylene carbonate (EC) and dimethyl carbonate (DMC) in a volume ratio of 1:1.
[0030] Performance Characterization:
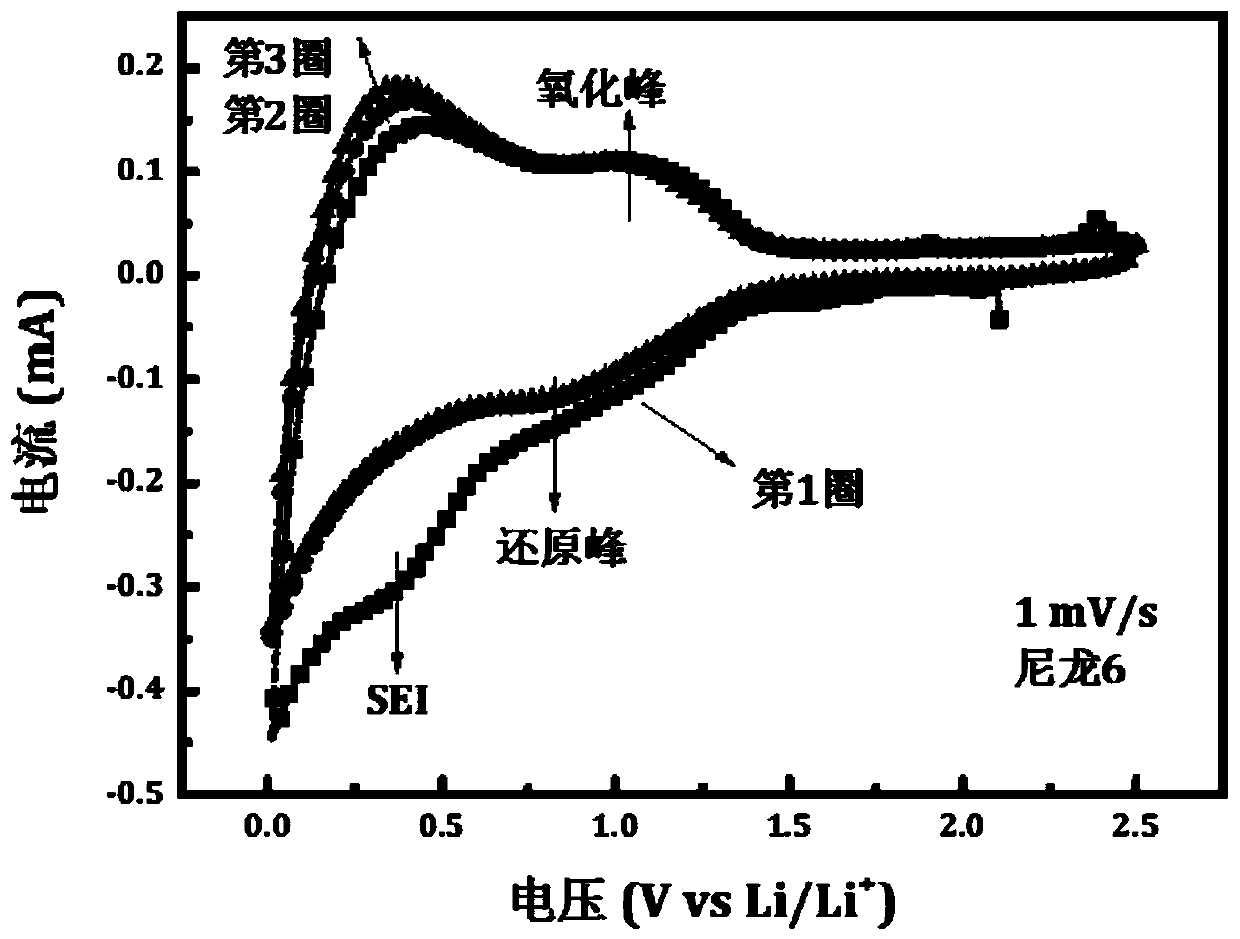
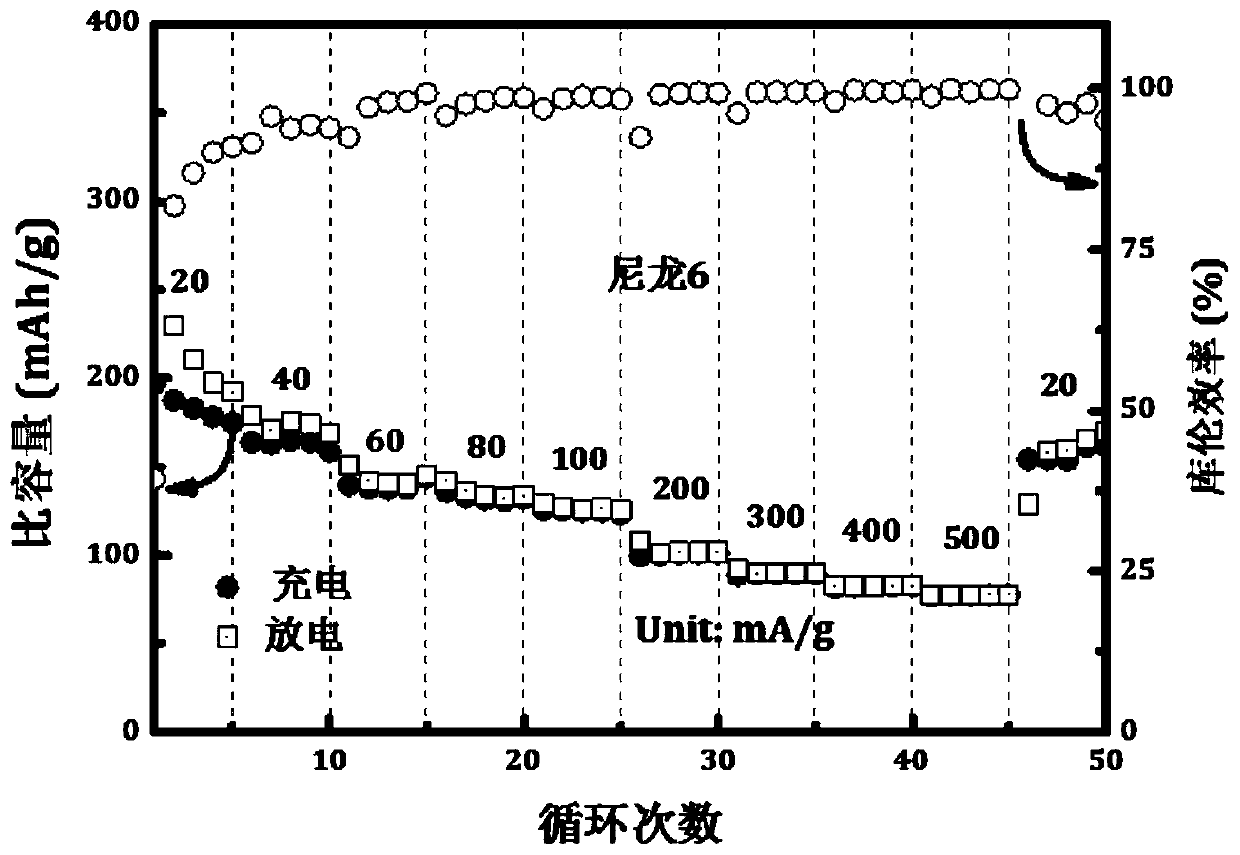
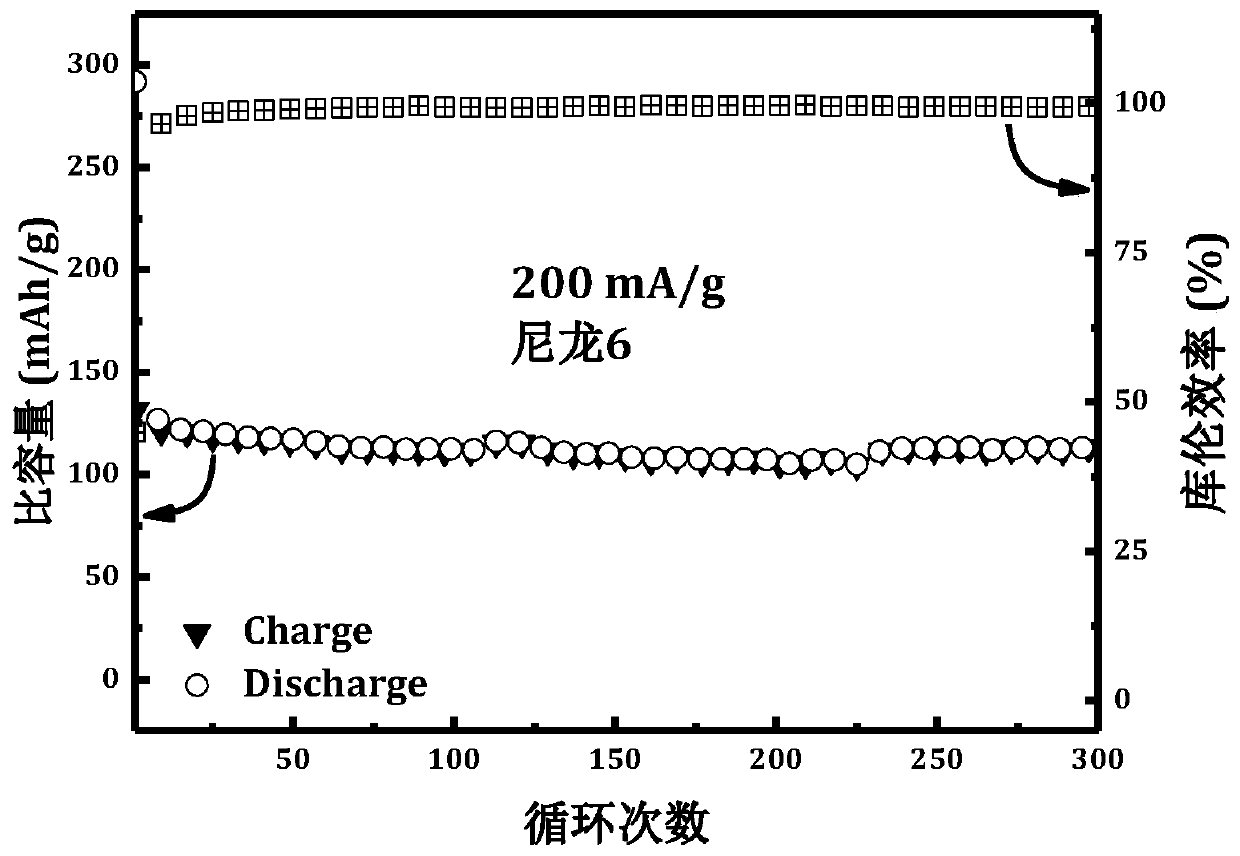
[0031] Such as figure 1 As shown, i...
Embodiment 2
[0035] In the second embodiment, nylon 66 / graphene composite material is used as the electrode active material.
[0036] Preparation:
[0037]Take 4.8g of commercially available nylon 66 powder and 0.2g of graphene, add 20mL of N-methylpyrrolidone, mix well, transfer to a ball mill jar, and go through a high-speed mechanical ball mill for 6 hours at a speed of 200rpm. Washing with ethanol and drying in vacuum to obtain the nylon 66 / graphene composite electrode active material.
[0038] Used in batteries:
[0039] Mix and grind the nylon 66 / graphene composite electrode active material prepared in the above steps with conductive agent (SP) and polyvinylidene fluoride (PVDF) evenly according to the mass ratio of 6:3:1, and add an appropriate amount of N-methyl - 2-Pyrrolidone (NMP), ground and stirred into a stable slurry. Coat it into a 0.06mm-thick pole piece, dry it in a blast drying oven at 60°C for 3-6 hours, then move it into a vacuum drying oven and dry it at 80°C for 1...
Embodiment 3
[0045] The method for providing nylon 610 / carbon fiber composite electrode active material in the present embodiment three is specifically:
[0046] Take 4.5g of commercially available nylon 610 powder and 0.5g of carbon fiber, add 50mL of N,N-dimethylformamide, mix evenly, and undergo high-speed shearing for 2 hours at a speed of 20,000rpm. Suction filtration, washing with ethanol, and vacuum drying to obtain the nylon 610 / graphene composite electrode active material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com