Polyarylether anion-exchange membrane material containing n-spirocyclic quaternary ammonium salt group in side chain and preparation method thereof
A technology of anion exchange membrane and spirocyclic quaternary ammonium salt, which is applied in the field of polyarylether anion exchange membrane materials and its preparation, and can solve the problems of reducing ion conductivity and losing cationic groups in polymer chains
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
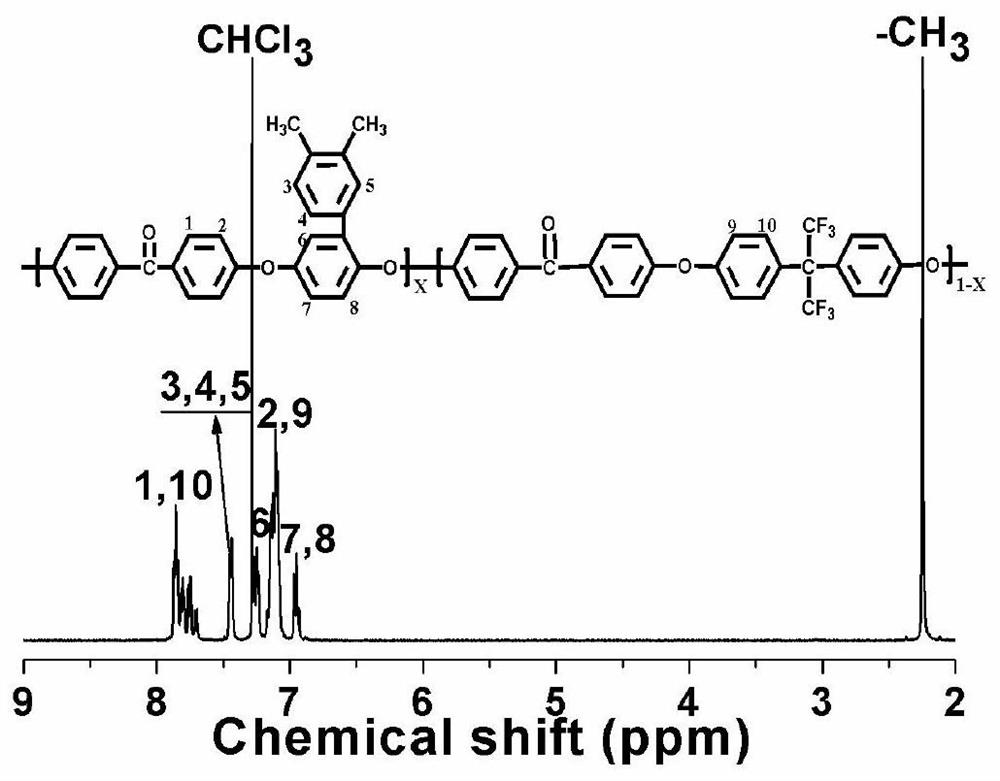
[0044] Under the condition of high-purity nitrogen protection, 6.4198g (0.0300mol) of 3,4-dimethylphenylhydroquinone, hexafluorobisphenol A6 .7175g (0.0200mol), 4,4-difluorobenzophenone 10.8885g (0.0500mol), sulfolane 57mL (solid content 25%), toluene 25mL and potassium carbonate 7.5824g (0.055mol), reacted at 140°C for 5 hours, then distilled toluene, raised the temperature to 210°C to continue the reaction, observed the change of the viscosity of the system, and stopped the reaction after the viscosity of the system increased sharply. The polymerized reaction product was discharged in distilled water, boiled and washed 9 times repeatedly with distilled water, and dried in an oven for 48 hours to obtain a polyaryletherketone polymer containing hexafluorobisphenol A in the main chain and containing methylphenyl in the side chain (X=0.6, Y=0.4), the yield was 98%.
Embodiment 2
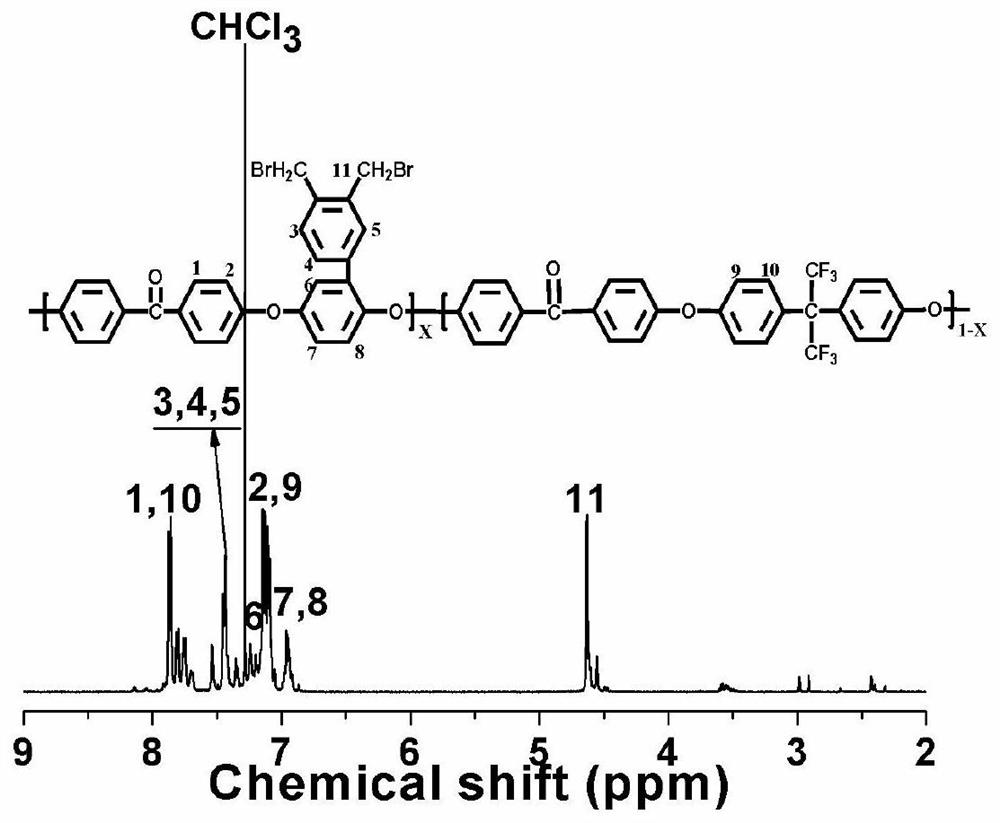
[0046] Under the protection of high-purity nitrogen, 5.0000 g (11.3430 mmol) of polyetheretherketone polymer containing methylphenyl in the side chain and 1,1,2,2-tetra Dissolve 50mL of ethyl chloride, add 1.4537g (8.1670mmol) of N-bromosuccinimide and 0.1976g (0.8167mmol) of dibenzoyl peroxide, heat up to 70°C under nitrogen protection, and react for 10 hours , the polymerization reaction product was precipitated in absolute ethanol, crushed, heated and refluxed with absolute ethanol for 9 times, and dried in an oven for 48 hours to obtain the main chain containing hexafluorobisphenol A side chain containing bromomethylphenyl polyarylether Ketone polymer (X=0.6, Y=0.4), yield 95%.
Embodiment 3
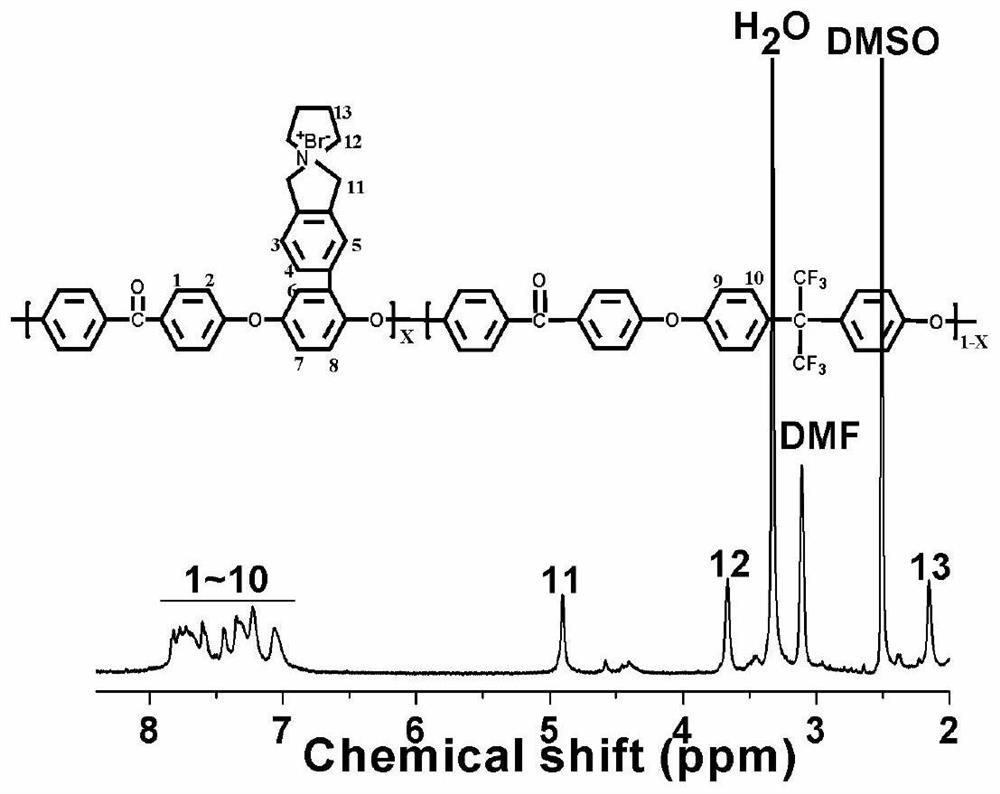
[0048] Add 2.5000 g (4.7405 mmol) of bromomethylphenyl polyetheretherketone polymer and 250 mL of N, N-dimethylformamide into a 500 mL three-necked flask equipped with mechanical stirring and nitrogen protection device, and dissolve N, 473uL (2.8443mmol) of N-diisopropylethylamine and 236uL (2.8443mmol) of tetrahydropyrrole were dissolved in 3mL of N,N-dimethylformamide, placed in a constant pressure dropping funnel and slowly added dropwise to the bromine-containing In the N,N-dimethylformamide solution of methylphenyl polyetheretherketone polymer, stir for 30 minutes, then heat up to 60°C, react for 12 hours, filter, cast and lay a film on a glass plate, dry the film Immerse in 1M NaOH aqueous solution, place at room temperature for 48 hours, take out the film, wash in deionized water until neutral, store in deionized water, and obtain the main chain containing hexafluorobisphenol A side chain containing five-membered N-spiro ring quaternary ammonium salt The polyaryletherke...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com