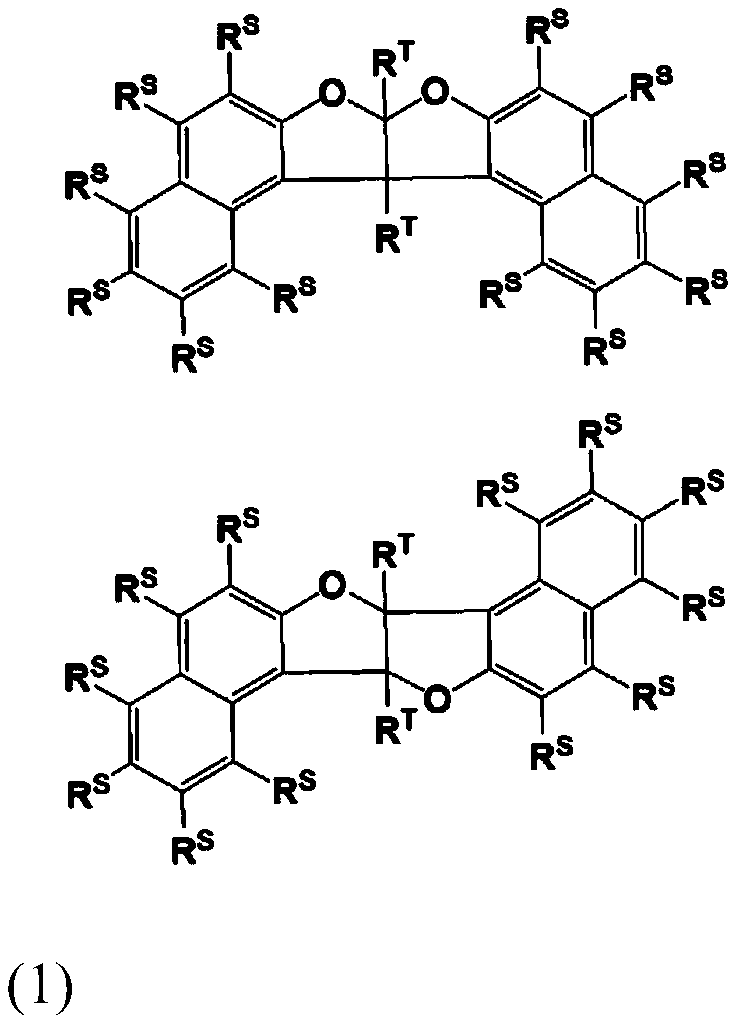
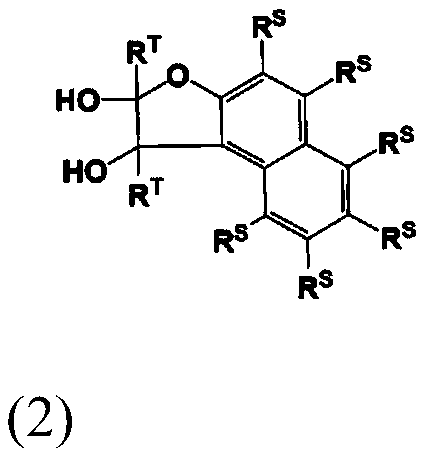
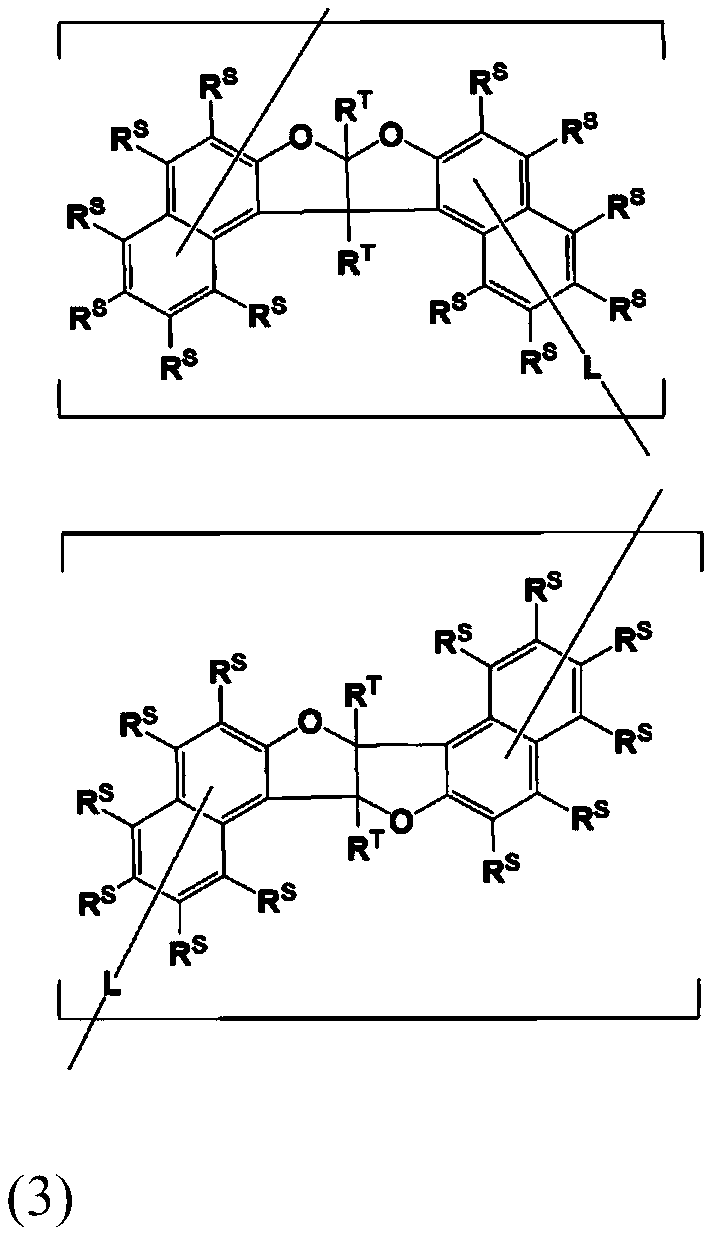
Compound, resin, composition, resist pattern forming method and pattern forming method
A technology of compounds and compositions, which is applied in the fields of compounds, resins, compositions, and resist pattern formation and pattern formation, can solve problems such as difficult to obtain resist pattern film thickness, and achieve good formability and good resist pattern High effect of shape and solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0342] Hereinafter, this embodiment will be described in more detail with reference to synthesis examples and examples, but this embodiment is not limited to these examples at all.
[0343] (carbon concentration and oxygen concentration)
[0344] Carbon concentration and oxygen concentration (mass %) were measured by organic elemental analysis using the following apparatus.
[0345] Device: CHNCORDERMT-6 (manufactured by YanacoCo., Ltd.)
[0346] (molecular weight)
[0347] The molecular weight of the compound was measured by LC-MS analysis using Acquity UPLC / MALDI-Synapt HDMS manufactured by Water Corporation.
[0348] In addition, gel permeation chromatography (GPC) analysis was carried out under the following conditions to obtain polystyrene-equivalent weight average molecular weight (Mw), number average molecular weight (Mn), and degree of dispersion (Mw / Mn).
[0349] Device: Shodex GPC-101 (manufactured by Showa Denko Co., Ltd.)
[0350] Column: KF-80M×3
[0351] Elu...
Synthetic example 1
[0379] A four-neck flask with an internal volume of 10 L and a detachable bottom equipped with a serpentine condenser, a thermometer, and a stirring blade was prepared. In this four-necked flask, 1.09 kg (7 mol, manufactured by Mitsubishi Gas Chemical Co., Ltd.) of 1,5-dimethylnaphthalene was added to 2.1 kg (28 mol as formaldehyde) of 40% by mass formalin solution in a nitrogen stream. , manufactured by Mitsubishi Gas Chemical Co., Ltd.) and 0.97 mL of 98% by mass sulfuric acid (manufactured by Kanto Chemical Co., Ltd.), were reacted under normal pressure at 100° C. for 7 hours while refluxing. Thereafter, as a diluting solvent, 1.8 kg of ethylbenzene (Special grade manufactured by Wako Pure Chemical Industries, Ltd.) was added to the reaction liquid, and after standing still, the aqueous phase of the lower layer was removed. Further, neutralization and water washing were performed, and ethylbenzene and unreacted 1,5-dimethylnaphthalene were distilled off under reduced pressu...
Embodiment 1~2、 Embodiment 1A~2A
[0383] (Examples 1-2, Examples 1A-2A, Comparative Example 1)
[0384] With regard to the aforementioned BiF-1, BiF-3, and CR-1, the solubility of each was evaluated. The results are shown in Table 1.
[0385] Separately, underlayer film-forming materials for lithography having the compositions shown in Table 1 were prepared. Next, the material for forming an underlayer film for lithography was spin-coated on a silicon substrate, and then baked at 240°C for 60 seconds, and further baked at 400°C for 120 seconds to form an underlayer film with a film thickness of 200 nm. . For acid generators, crosslinking agents and organic solvents, the following were used.
[0386] Acid generator: Di-tert-butyldiphenyliodonium nonafluoromethanesulfonate (DTDPI) manufactured by Midori Kagaku Co., Ltd.
[0387] ・Crosslinking agent: NIKALAC MX 270 (Nikalac) manufactured by Sanwa Chemical Co., Ltd.
[0388] Organic solvent: propylene glycol monomethyl ether acetate (PGMEA)
[0389] [etching...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com