A kind of composite bimetallic boride alkaline aqueous electrolyte system battery cathode material and preparation method thereof
A composite bimetallic and alkaline aqueous solution technology, which is applied in the direction of metal borides, battery electrodes, boron/borides, etc., can solve the problems of weak high-current charge and discharge capabilities, low utilization of active materials, and poor cycle performance of nickel electrodes. , to achieve the effect of increasing electronic conductivity, good initial charge and discharge performance and cycle performance, and excellent charge and discharge performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
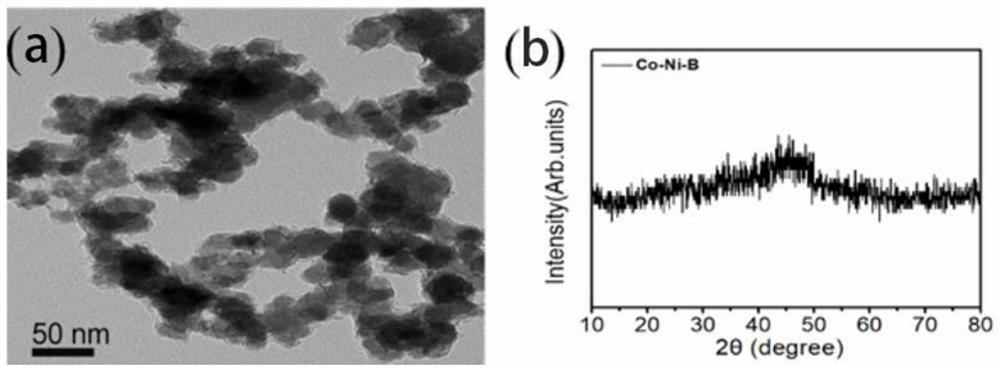
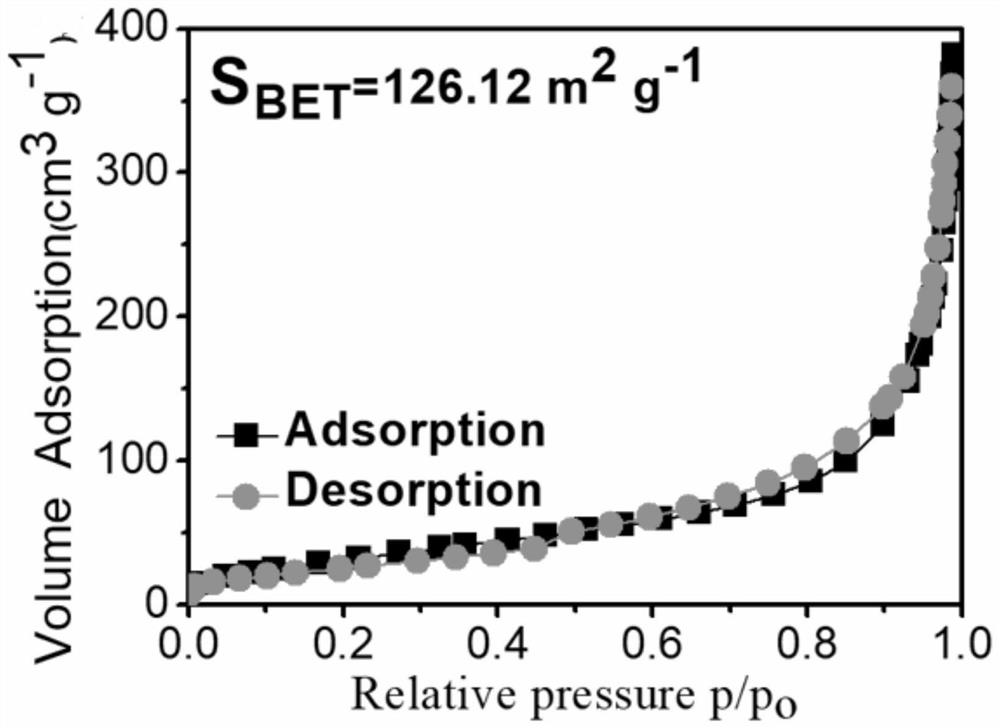
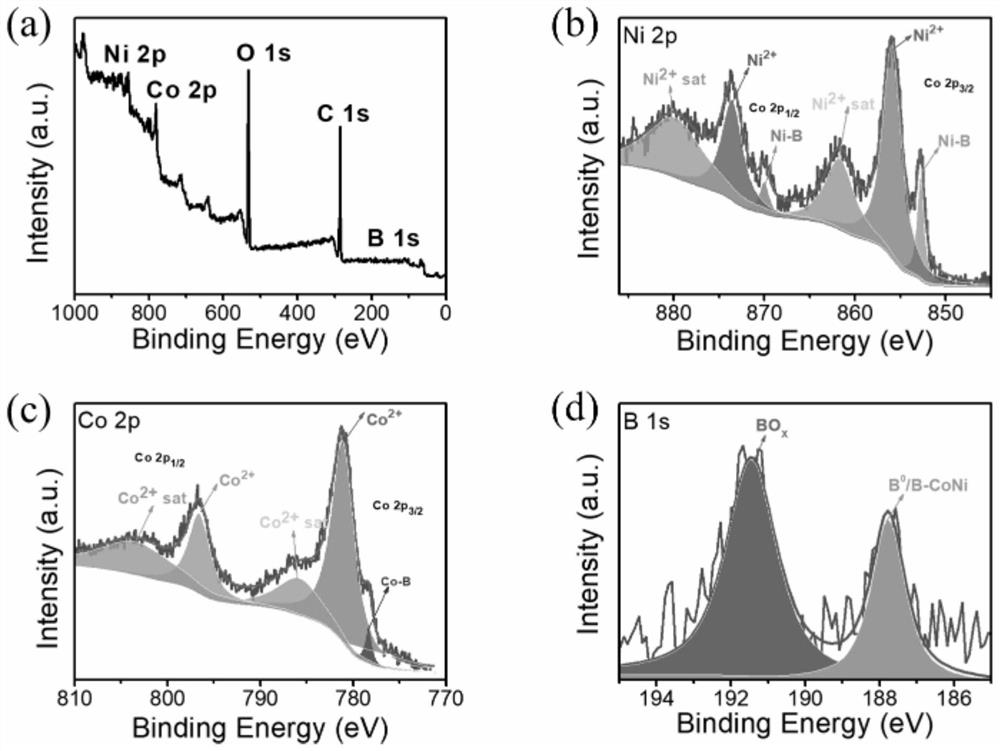
[0037] (1) 0.0025 mol of cobalt chloride, 0.0025 mol of nickel chloride and 5.0 g of polyethylene glycol were dissolved in 100 ml of deionized water, 20 ml of cyclohexane was added to this solution, and the mixture thus obtained was subjected to ultrasound at 293K Treated for 30 minutes to form a uniformly dispersed microemulsion. Then, 0.001 mol of sodium hydroxide was dissolved in 10 ml of deionized water (to form an alkaline solution with a pH value of about 13), and 0.02 mol of sodium borohydride was added to form a sodium borohydride solution. The sodium borohydride solution was added dropwise to the water-in-oil microemulsion under vigorous stirring, and when the bubbles stopped, the black precipitate in the solution was filtered and thoroughly washed with deionized water and ethanol. The washed powder is then dried in a nitrogen atmosphere to finally obtain a composite bimetallic boride.
[0038] The test results of the full-spectrum direct reading plasma emission spec...
Embodiment 2
[0045] 0.0015mol of cobalt chloride, 0.0035mol of nickel chloride and 5.0g of polyethylene glycol (M w = 20,000) was dissolved in 100 ml of deionized water, 20 ml of cyclohexane was added to this solution, and the mixture thus obtained was sonicated at room temperature for 40 minutes to form a water-in-oil microemulsion. Then 0.02mol of sodium borohydride was dissolved in 10ml of 0.1M sodium hydroxide solution (pH value was about 13) to prepare a sodium borohydride solution. The sodium borohydride solution was added dropwise to the oil-in-water microemulsion under vigorous stirring, and when the bubbles stopped, the black precipitate in the solution was filtered and thoroughly washed with deionized water and ethanol. Then, the washed powder is dried under vacuum to finally obtain a composite bimetallic boride;
[0046] The prepared product, acetylene black and polytetrafluoroethylene (PTFE) aqueous solution were mixed in a mass ratio of 8:1:1 to prepare a working electrode sl...
Embodiment 3
[0049] 0.0035mol of cobalt chloride, 0.0015mol of nickel chloride and 5.0g of polyethylene glycol (M w = 20,000) was dissolved in 100 ml of deionized water, 20 ml of cyclohexane was added to this solution, and the mixture thus obtained was sonicated at room temperature for 40 minutes to form a water-in-oil microemulsion. Then, 0.02 mol of sodium borohydride was dissolved in 10 ml of sodium hydroxide solution with a concentration of 0.1 M (pH value was about 13) to prepare a sodium borohydride solution. The sodium borohydride solution was added dropwise to the oil-in-water microemulsion under vigorous stirring, and when the bubbles stopped, the black precipitate in the solution was filtered and thoroughly washed with deionized water and ethanol. Then, the washed powder is dried under vacuum to finally obtain a composite bimetallic boride;
[0050] The prepared product, acetylene black and polytetrafluoroethylene (PTFE) aqueous solution were mixed in a mass ratio of 8:1:1 to pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com