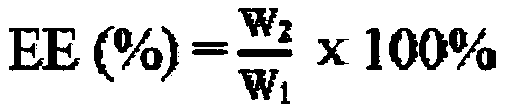Photosensitizer-releasing antibacterial nano micelles and preparation method and application thereof
A technology of nano micelles and photosensitizers, applied in the field of nano biomedical materials, can solve the problems of limited application of vancomycin, low encapsulation efficiency and drug loading rate, and it is difficult for vancomycin to achieve ideal drug efficacy, etc. Degradability, good biocompatibility, and the effect of prolonging circulation time in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Synthesis of mPEG-PCL-PAE
[0047] The synthesis of mPEG-PCL-PAE includes two parts, one, mPEG-PCL is obtained by ring-opening polymerization (ROP). Among them, mPEG-OH is used as the initiator, stannous isooctanoate (Sn(Oct) 2 ) as a catalyst and ε-CL as a monomer. The specific steps are as follows: weigh mPEG (4.0g, 2.0mmol) and ε-CL (10.0g, 87.8mmol) and dissolve them in 40mL of anhydrous toluene, add a drop of Sn(Oct) 2 , and then freeze the reaction liquid with liquid nitrogen, vacuumize, pass nitrogen, thaw, repeat three times, and exhaust the oxygen. Stir the reaction overnight at 110°C, then remove the solution, and dissolve the crude product in dichloromethane, then add glacial ether ten times the volume of dichloromethane to precipitate to remove impurities, filter and wash the solid product mPEG-PCL Dry in a vacuum oven and store at 4°C for later use. 2. Dissolve mPEG-PCL (1g) and triethylamine (2g) in a 250mL round bottom flask with 10mL of dichlorometha...
Embodiment 2
[0049] Preparation and characterization of mPEG-PCL-PAE-EA micelles Precisely weigh 100mg of mPEG-PCL-PAE and 20mg of escarcin A (EA) into a 25mL round bottom flask, add 15mL of acetone, and dissolve them by ultrasonication. Rotary evaporate at 40°C for about 30min until the acetone is volatilized and a transparent and uniform film is formed. Add 1 mg of D-mannitol, add double distilled water or replace it with 8mL of PBS (pH5.5, 6.5, 7.4), and place in a water bath at 55°C Rotate slowly to hydrate the film. After hydration for 3 hours, use a probe-type ultrasonic instrument to sonicate for 20 minutes (20HZ) in an ice-water bath to obtain drug-loaded nanomicelles ( figure 1 A), use a 0.22 μm filter to remove unencapsulated EA, and freeze-dry the micelles at -80°C for 48 hours in a vacuum to make a freeze-dried powder ( figure 1 B) Store in a refrigerator at 4°C. The micelles made with 5% D-mannitol as a freeze-drying protective agent have good resolubility after freeze-drying...
Embodiment 3
[0052] The preparation process of embodiment 3 is the same as that of embodiment 2, and the difference is that, by weight, photosensitizer scutoclavin B 5 parts, monomethoxy polyethylene glycol-polycaprolactone-poly β-amino ester 5 parts 0.01 part of freeze-dried excipient sucrose; the organic solvent was replaced by dichloromethane; the rotary evaporation temperature was 40°C, and the rotary evaporation was 2h; the hydration temperature was 40°C, and the hydration time was 6h; , the frequency is 20HZ; the freeze-drying condition is -50℃, 48h.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com