A kind of preparation method of highly active iridium-zirconium composite oxide inert anode
A composite oxide, inert anode technology, applied in electrodes, liquid chemical plating, coatings, etc., can solve the problems of limited large-scale application, high anode production cost, short anode life, etc., to achieve easy operation, equipment Few and simple, low equipment investment effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
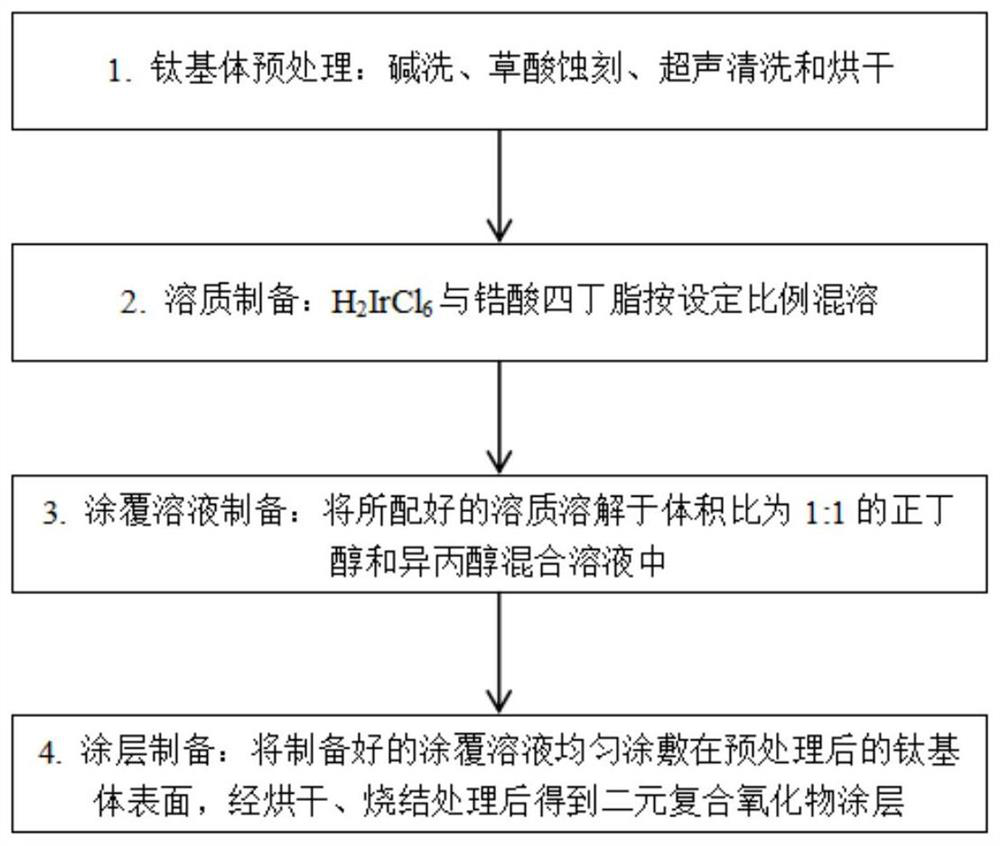
[0053] First, a titanium plate of 10mm×10mm×1mm was washed with alkali at 60°C for 30 minutes, etched with 10% oxalic acid at 90°C for 2.5 hours, ultrasonically cleaned with deionized water for 10 minutes, and then dried for use.
[0054] Weigh a certain mass of H 2 IrCl 6 Miscible with tetrabutyl zirconate.
[0055] The miscible H 2 IrCl 6 The mixed solution of tetrabutyl zirconate and tetrabutyl zirconate is dissolved in a mixed solvent of n-butanol and isopropanol mixed in a ratio of 1:1 to obtain a coating solution. H in the coating solution 2 IrCl 6 The molar concentrations of zirconate and tetrabutyl zirconate were 0.18 and 0.02 mol / L, respectively.
[0056] The prepared coating solution was evenly coated on the surface of the pretreated titanium substrate with a brush, dried at 120°C for 10 minutes, then sintered at 450°C for 10 minutes, and cooled to room temperature after taking it out. After the above steps were repeated 25 times, the anode plate was sintered ...
Embodiment approach 2
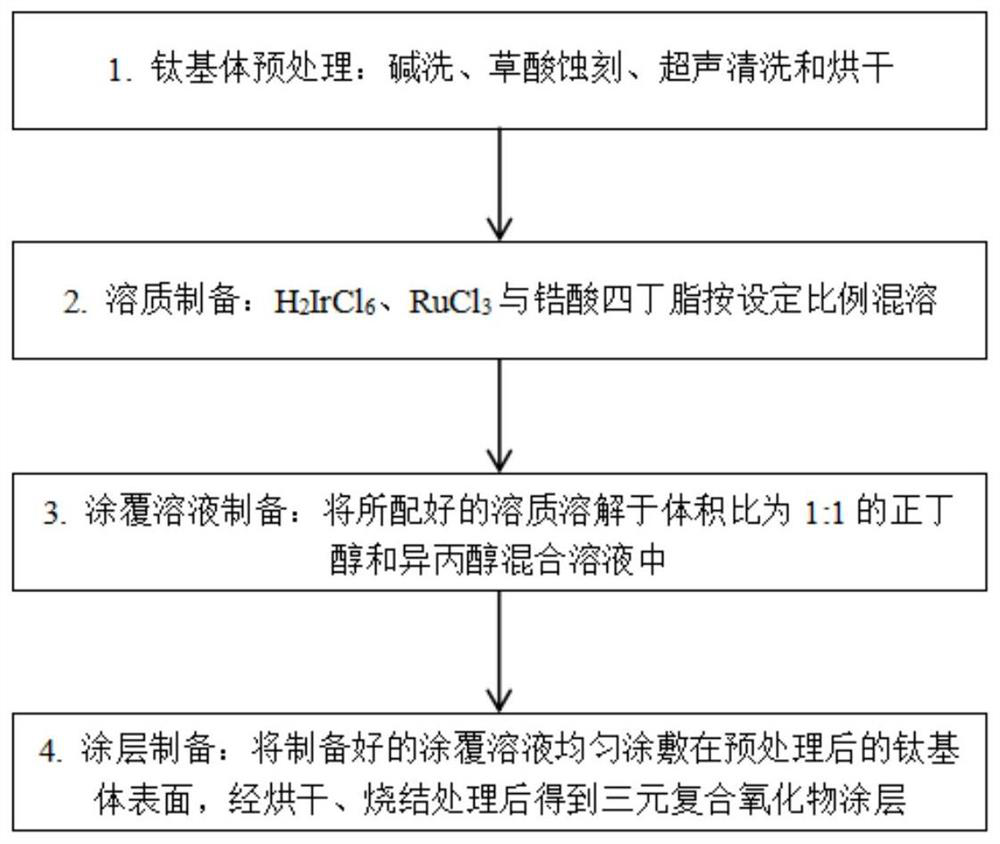
[0059] First, a titanium plate of 10mm×10mm×1mm was washed with alkali at 60°C for 30 minutes, etched with 10% oxalic acid at 90°C for 2.5 hours, ultrasonically cleaned with deionized water for 10 minutes, and then dried for later use.
[0060] Weigh a certain mass of H 2 IrCl 6 Miscible with tetrabutyl zirconate.
[0061] H 2 IrCl 6 The mixed solution with tetrabutyl zirconate is dissolved in a mixed solvent of n-butanol and isopropanol mixed in a ratio of 1:1 to obtain a coating solution. H in the coating solution 2 IrCl 6 The molar concentrations of zirconate and tetrabutyl zirconate were 0.14 and 0.06 mol / L, respectively. .
[0062] The prepared coating solution was evenly coated on the surface of the pretreated titanium substrate with a brush, dried at 120°C for 15 minutes, then sintered at 500°C for 15 minutes, and cooled to room temperature after taking it out. After the above steps were repeated 25 times, the anode plate was sintered at 500° C. for 1 hour to ob...
Embodiment approach 3
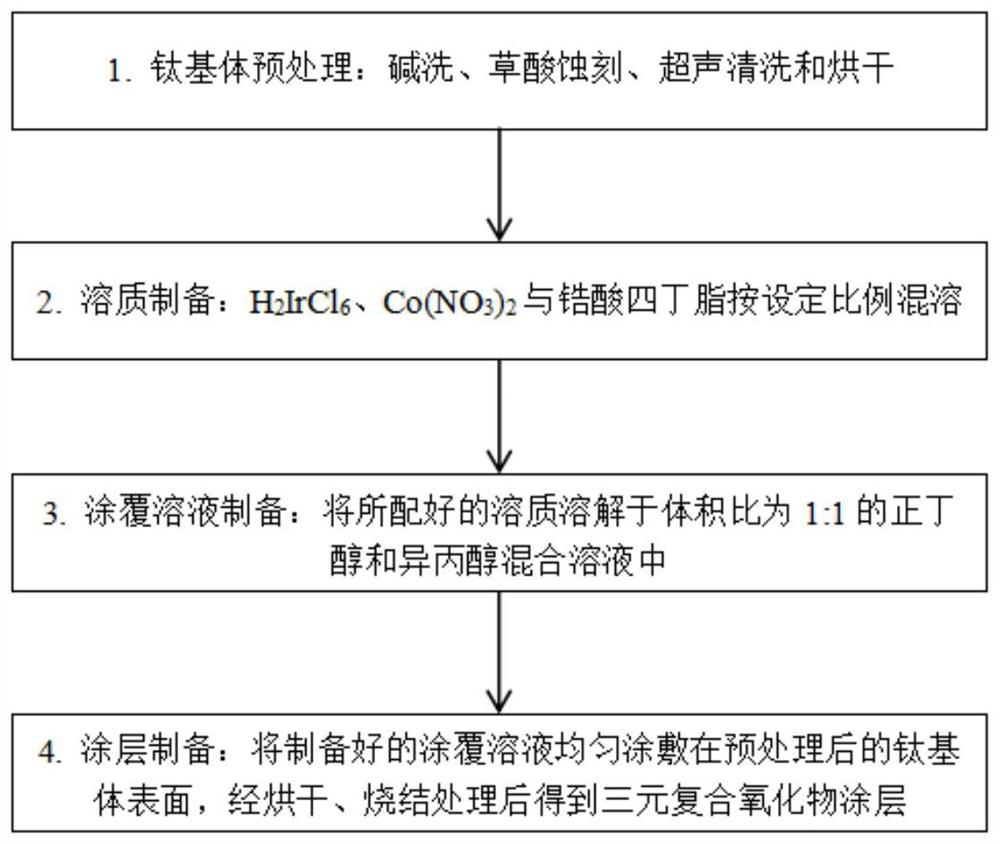
[0065] First, a titanium plate of 10mm×10mm×1mm was washed with alkali at 60°C for 30 minutes, etched with 10% oxalic acid at 90°C for 2.5 hours, ultrasonically cleaned with deionized water for 10 minutes, and then dried for later use.
[0066] Weigh a certain mass of H 2 IrCl 6 Miscible with tetrabutyl zirconate.
[0067] H 2 IrCl 6 The mixed solution with tetrabutyl zirconate is dissolved in a mixed solvent of n-butanol and isopropanol mixed in a ratio of 1:1 to obtain a coating solution. H in the coating solution 2 IrCl 6 The molar concentrations of tetrabutyl zirconate and tetrabutyl zirconate are both 0.10mol / L.
[0068] The prepared coating solution was evenly coated on the surface of the pretreated titanium substrate with a brush, dried at 120°C for 15 minutes, then sintered at 400°C for 15 minutes, and cooled to room temperature after taking it out. After the above steps were repeated 25 times, the anode plate was sintered at 400° C. for 1 hour to obtain a binar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com