A kind of star conjugated structure polymer and its preparation method and application
A conjugated structure and polymer technology, applied in the field of star-shaped conjugated structure polymers and their preparation, can solve the problems of low electrical performance of field effect transistor devices, and achieve good application prospects, simple synthesis routes, and strong practicability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] Another object of the present invention is to provide the preparation method of above-mentioned polymer, comprises the following steps:
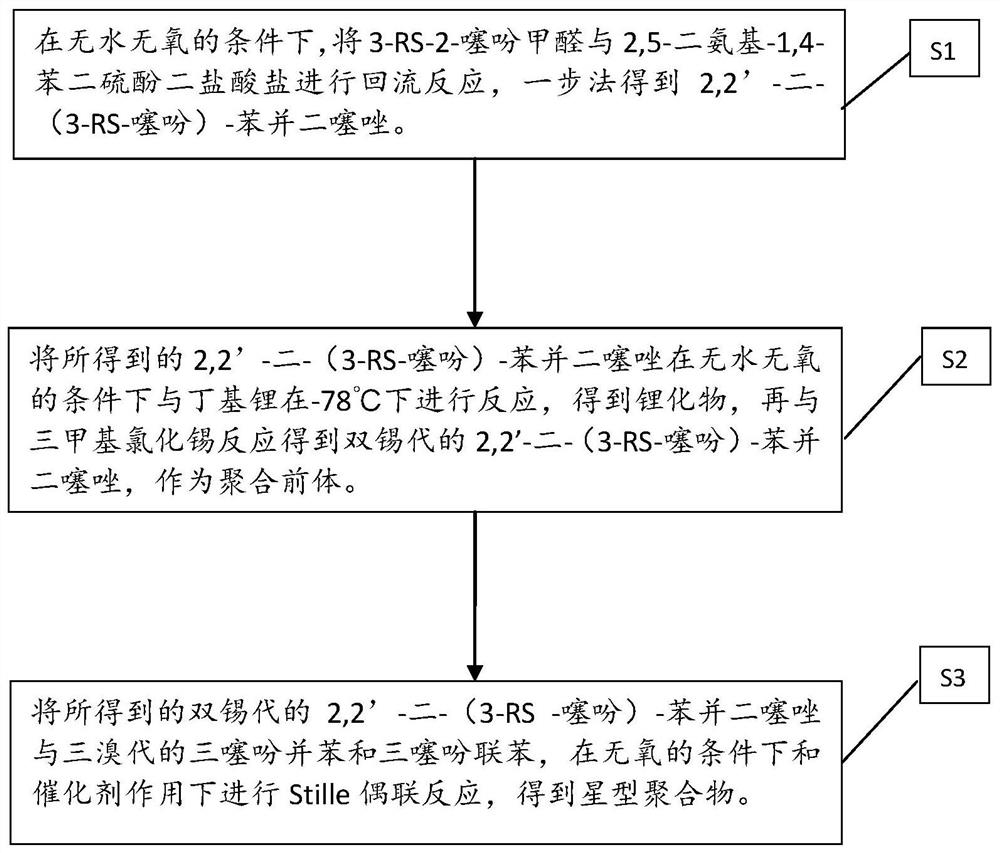
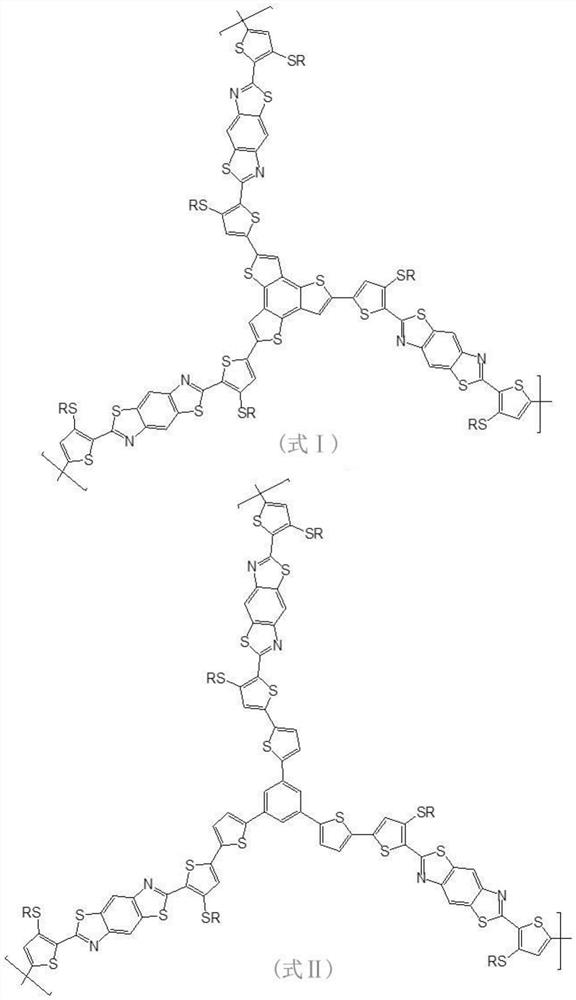
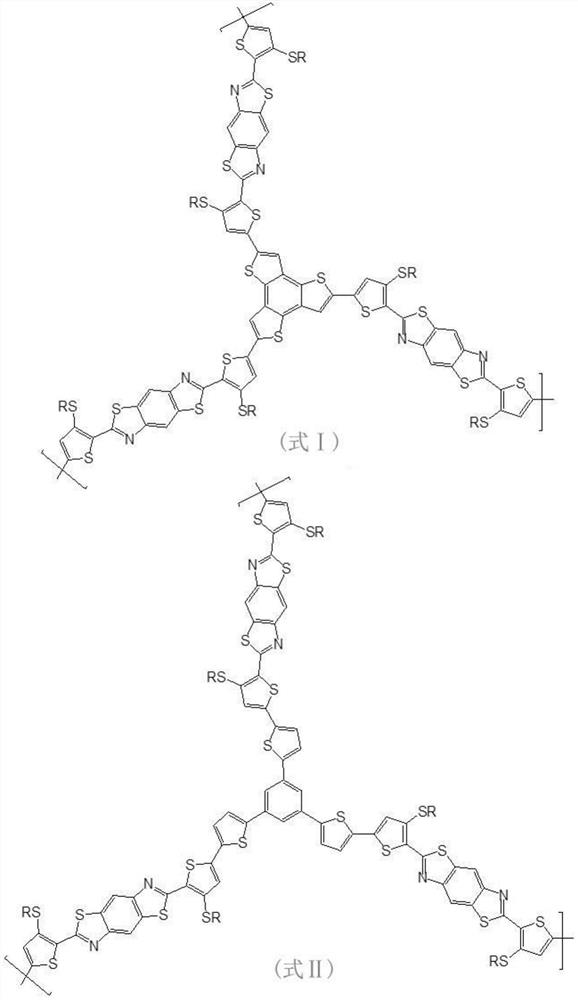
[0042] Step S1, under anhydrous and oxygen-free conditions, reflux reaction of 2,5-diamino-1,4-benzenedithiol dihydrochloride and 3-RS-2-thiophenecarbaldehyde under piperidine conditions, One-step method to obtain 2,2'-bis-(3-RS-thiophene)-benzobithiazole;
[0043] Step S2, reacting the 2,2'-bis-(3-RS-thiophene)-benzobithiazole obtained in step S1 with butyllithium (1.6M) at -78°C under anhydrous and oxygen-free conditions , to obtain a lithium compound, and then react with trimethyltin chloride to obtain a bistin-substituted 2,2'-bis-(3-RS-thiophene)-benzobithiazole as a polymerization precursor ①;
[0044] Step S3, combining the bistin-substituted 2,2'-bis-(3-RS-thiophene)-benzobithiazole and tribromotrithienoacene and / or tribromotrithiophene obtained in step S2 Biphenyl (polymerization precursor 2.) carries out Stille coupling re...
Embodiment 1
[0057] Embodiment 1, the synthesis of 2,2'-two-(3-dodecylthio-thiophene)-benzobithiazole
[0058] In a 100mL round bottom flask, add 3-dodecylthio-2-thiophenecarbaldehyde (50mmol, 14.0g) and 2,5-diamino-1,4-benzenedithiol dihydrochloride (20mmol, 2.8 g), add 50mL of anhydrous tetrahydrofuran after filling with nitrogen, then add 10mL of piperidine, heat and reflux for 24 hours, cool to room temperature, pour into water, filter the precipitated solid, wash with water, and wash with methanol. The obtained crude product was purified by silica gel column, petroleum ether: ethyl acetate was used as eluent, and the solvent was spin-dried to obtain brown yellow solid 2,2'-bis-(3-dodecyl-thiophene)-benzobis Thiazole, the yield was 48.6%.
[0059] The brownish yellow solid was detected by mass spectrometry and nuclear magnetic resonance: MS (Maldi-TOF) m / z: 756 (M+); 1 H NMR (400MHz, CD 2 Cl 2 ppm): δ=8.20(s,2H), 7.18(d,2H), 6.68(d,2H), 2.57(t,24H), 1.63(m,4H), 1.34-1.26(m,36H), 0...
Embodiment 2
[0060] Example 2, the synthesis of 2,2'-two-(3-dodecylsulfanyl-5-(trimethyltin)-thiophene)-benzobithiazole
[0061] In a 250mL three-necked flask, add 2,2'-bis-(3-dodecylthio-thiophene)-benzobithiazole (5mmol, 3.78g) under a nitrogen atmosphere, add 80mL of dry tetrahydrofuran, and Slowly add butyl lithium (12mmol, 2.5M, 4.8mL) dropwise, continue to stir for 1 hour, add trimethyltin chloride (14mmol, 1M, 14mL), gradually rise to room temperature and react overnight, add saturated ammonium chloride solution, Extract with ether, wash with water, combine the organic phases, dry over anhydrous magnesium sulfate, filter and spin off the solvent. The obtained crude product was purified by a neutral alumina column, petroleum ether: ethyl acetate was used as eluent, and the solvent was spin-dried to obtain a light yellow solid 2,2'-bis-(3-dodecylthio-5- (Trimethyltin)-thiophene)-benzobithiazole, the yield was 62.6%.
[0062] The light yellow solid was detected by mass spectrometry, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com