Method for leaching vanadium by anode electrolysis in strong alkali electrolyte solution
A technology of strong alkali electrolyte and anode electrolysis, which is applied in the field of leaching vanadium from vanadium iron spinel minerals by electrolysis, which can solve the problems of increasing production costs, increasing production costs, increasing energy consumption, etc., and achieves pollution reduction and convenient operation , to handle the effect of easy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
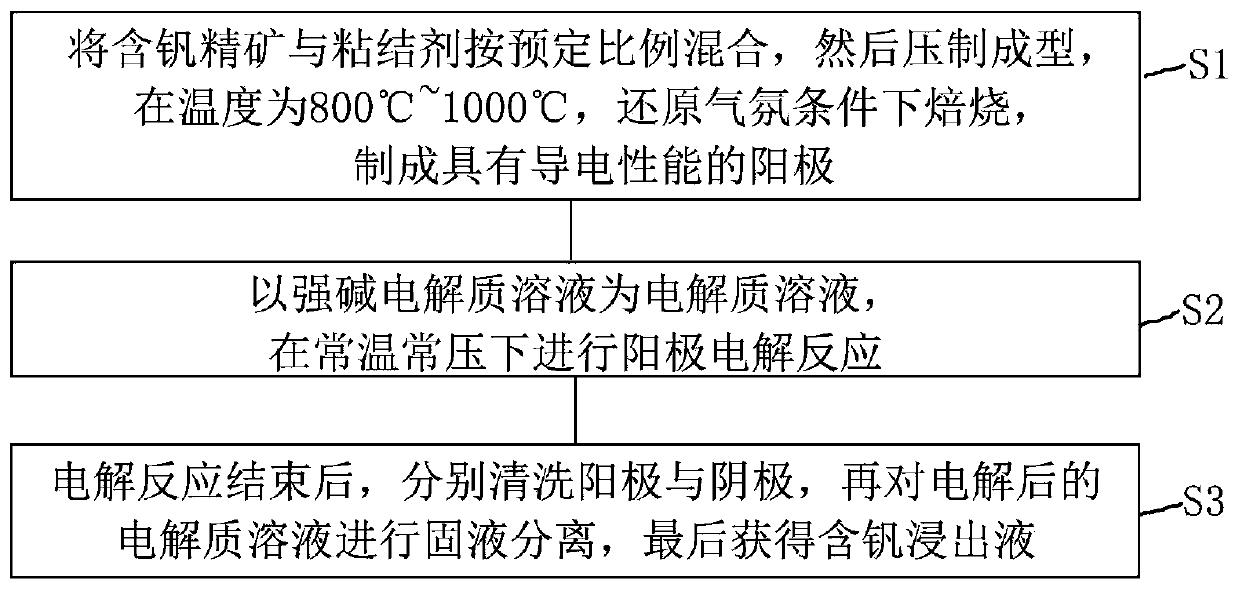
[0084] (1) Preparation of vanadium-iron spinel: using vanadium trioxide and ferric oxide as initial raw materials, roasting at 1200°C for 48 hours in a reducing atmosphere to obtain Fe 2 VO 4 .
[0085] (2) Using the pure vanadium-iron spinel obtained in step (1) as a raw material to make an anode, the diameter of the anode is 10mm, and the thickness is about 3mm.
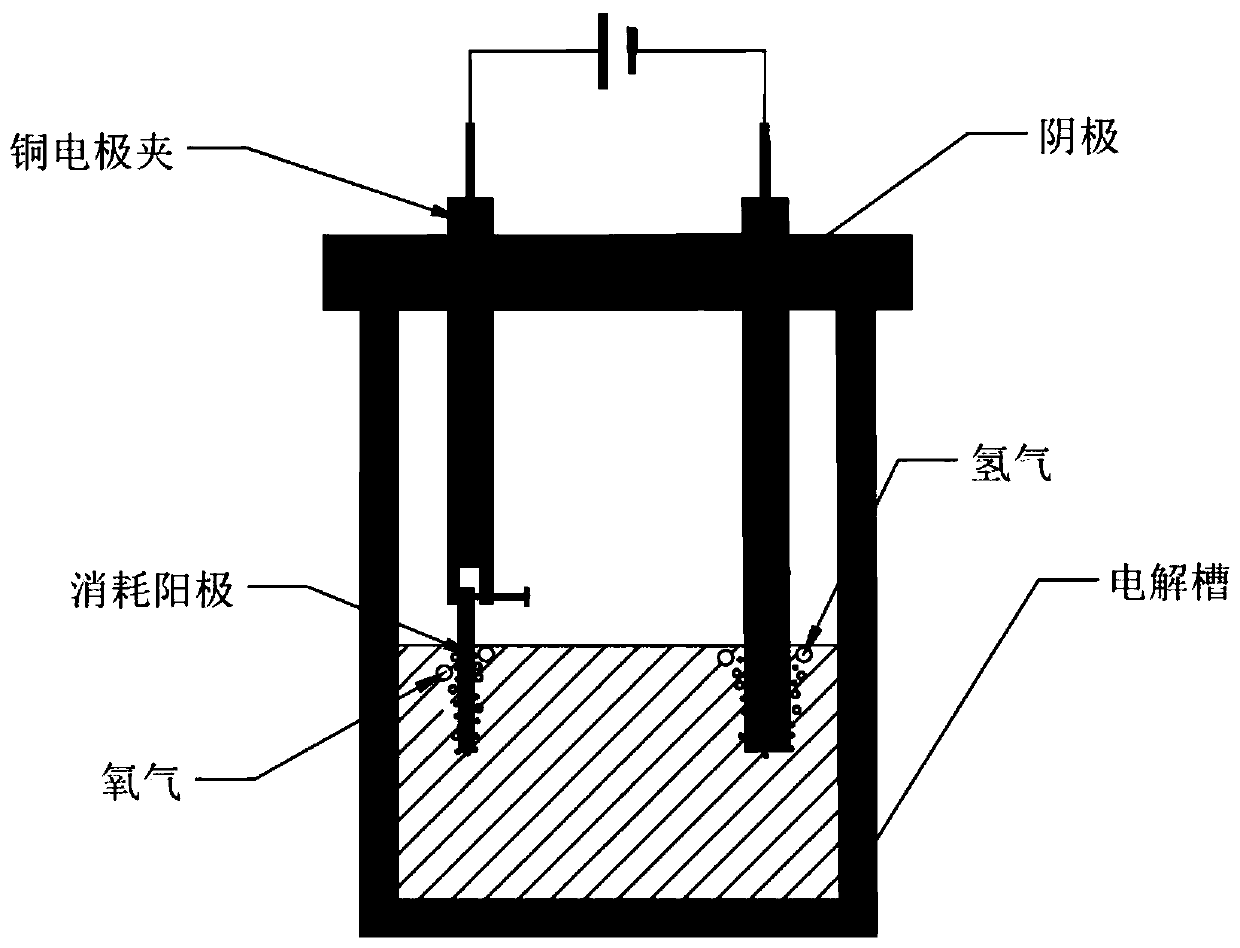
[0086] (3) The electrolytic reaction is carried out in an electrolytic cell at normal temperature and pressure. The cathode material of the electrolytic cell is a graphite rod with a diameter of 6mm, the center distance between the anode and the cathode is 5cm, and the electrolytic solution is 35% sodium hydroxide solution.
[0087] (4) The cell voltage is selected as 5V, the electromagnetic stirring speed is 500r / min, the electrolysis temperature is room temperature, and the electrolysis reaction is selected as 1h.
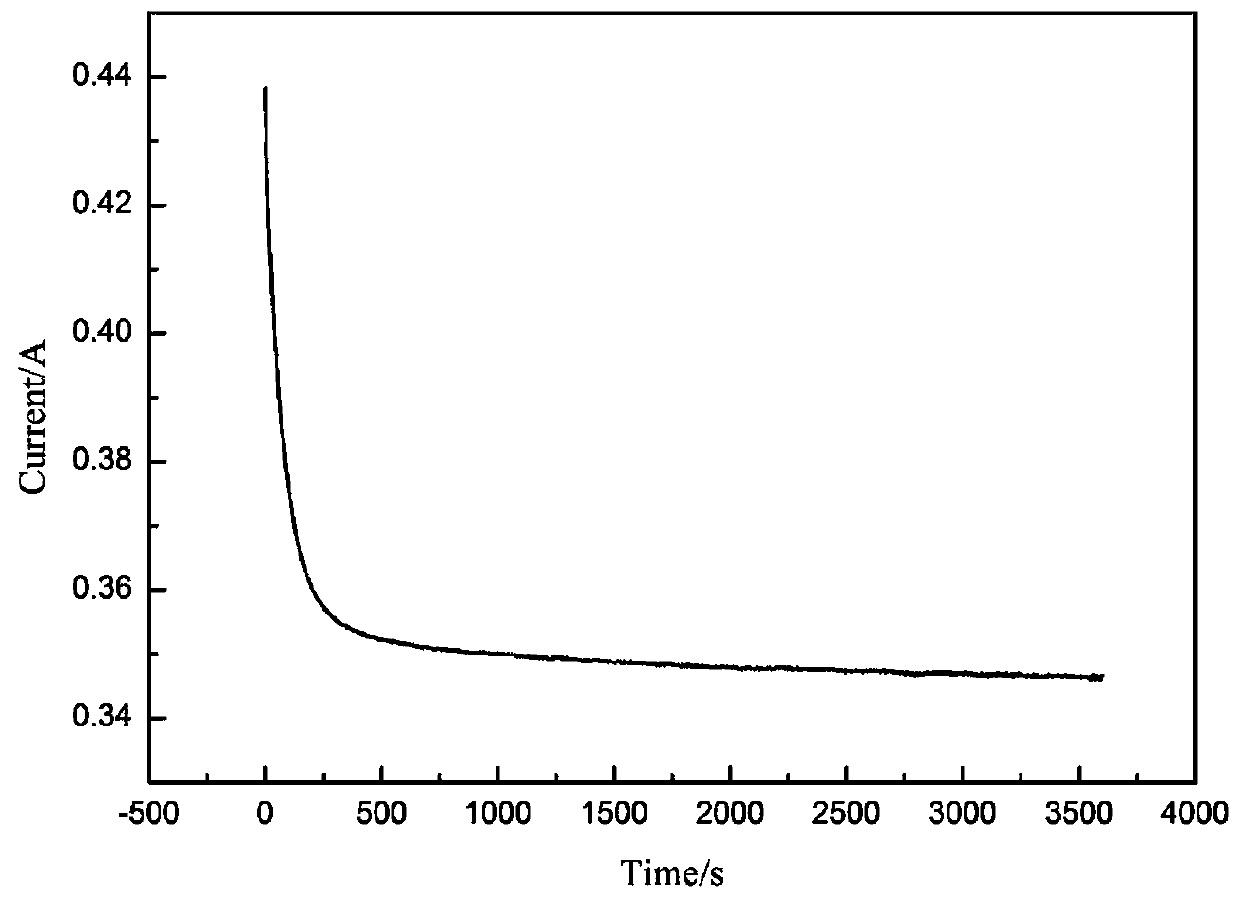
[0088] (5) After the electrolysis reaction starts, record the relationship between current and t...
Embodiment 2
[0090] (1) Using vanadium-iron-spinel-type vanadium concentrate as the initial raw material, ball mill the vanadium concentrate to a particle size of 0.074mm or less.
[0091] (2) take by weighing the vanadium-iron-spinel type vanadium concentrate and 0.4g pure vanadium-iron-spinel (Fe) after the ball milling 2 VO 4 ) in an agate mortar, add 3 drops of 5% polyvinyl alcohol solution (about 0.1 g) and mix well, then press it into a round block with a diameter of 10 mm and a thickness of about 3 mm.
[0092] (3) Place the produced round block in a high-temperature furnace, and bake it for 6 hours at a temperature of 800° C. under a reducing atmosphere to make an anode with electrical conductivity.
[0093] (4) electrolytic reaction is carried out in normal temperature and pressure electrolyzer, and the cathode material of described electrolyzer is the graphite rod of diameter 6mm, and the center-to-center distance between anode and cathode is 5cm, and electrolytic solution is 35% ...
Embodiment 3
[0097] (1) Using vanadium-iron-spinel-type vanadium concentrate as the initial raw material, ball mill the vanadium concentrate to a particle size of 0.074mm or less.
[0098] (2) Weigh 0.8g of ball-milled vanadium-iron-spinel-type vanadium concentrate in an agate mortar, add 3 drops of polyvinyl alcohol solution (about 0.1g) with a mass fraction of 5%, mix well, then press Form into a round block with a diameter of 10mm and a thickness of about 3mm.
[0099] (3) Place the produced round block in a high-temperature furnace, and bake it for 6 hours at a temperature of 800° C. under a reducing atmosphere to make an anode with electrical conductivity.
[0100] (4) The electrolytic reaction is carried out in an electrolytic cell at normal temperature and pressure; the cathode material of the electrolytic cell is a graphite rod with a diameter of 6mm, the center distance between the anode and the cathode is 5cm, and the electrolytic solution is 35% sodium hydroxide solution.
[01...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com