A kind of bilirubin nanoparticle for the treatment of acute pancreatitis and its preparation method
A technology of acute pancreatitis and nanoparticles, which is applied in the direction of medical preparations containing non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. Prepare nanoparticles and other problems to achieve the effect of prolonging the residence time, no toxic side effects, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation of embodiment 1 bilirubin nanoparticles
[0035] After silkworm cocoons are degummed with sodium bicarbonate weak alkali solution, they are completely dissolved with lithium bromide solution, and regenerated silk fibroin is obtained after dialysis with deionized water; 10 mg of bilirubin, 1 mg of genipin, and 10 mg of ceramide phosphoethanolamine are dissolved in 2 ml of DMSO , and then add 7ml of acetone to mix to obtain the organic phase; take 50mg of silk fibroin and dissolve it in 1ml of pure water, centrifuge at 4000rpm for 5min and take the supernatant as the aqueous phase; mix the aqueous phase and the organic phase at 50°C for 6h, dialyze Removing the organic solvent; diluting the obtained nanosuspension with pure water to obtain bilirubin nanoparticles ①.
[0036] The preparation method of bilirubin nanoparticles ② is the same as this method, the difference is that ceramide phosphoethanolamine is replaced with equal mass of ceramide phosphorylch...
Embodiment 2
[0046] The characterization of embodiment 2 bilirubin nanoparticles
[0047] 2.1 Particle size and Zeta potential
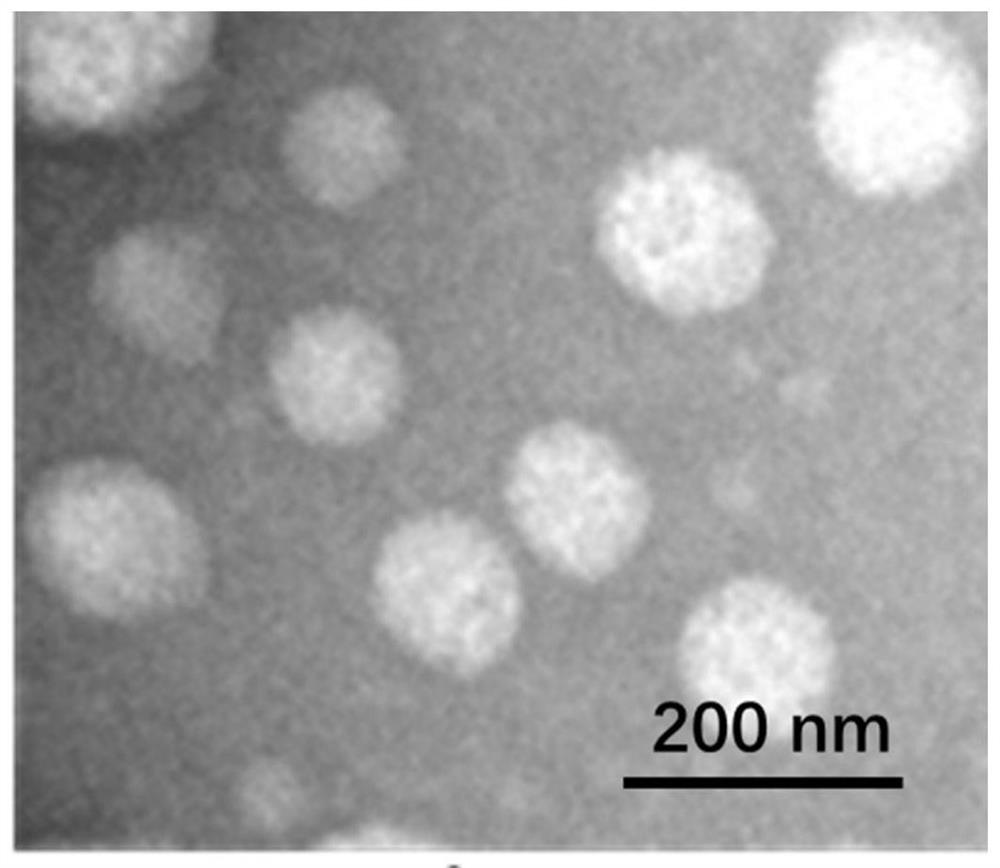
[0048]The bilirubin nanoparticles prepared in Example 1 were taken, and the average size and Zeta potential of the nanoparticles were detected by dynamic light scattering. The morphological examination of bilirubin nanoparticles① was carried out by transmission electron microscopy. Specifically, after diluting bilirubin nanoparticles to an appropriate concentration, the samples were prepared by dropping on a copper grid coated with a carbon film, and then examined under a transmission electron microscope. Observe the shape of the sample.
[0049] The particle size and Zeta potential are shown in Table 1. The results showed that the particle size of bilirubin nanoparticles ① was 184.34±10.78, and the PDI value was 0.083. The particle size of bilirubin nanoparticles② was 176.39±8.73, and the PDI value was 0.102. PDI represents the uniformity of the particle size...
Embodiment 3
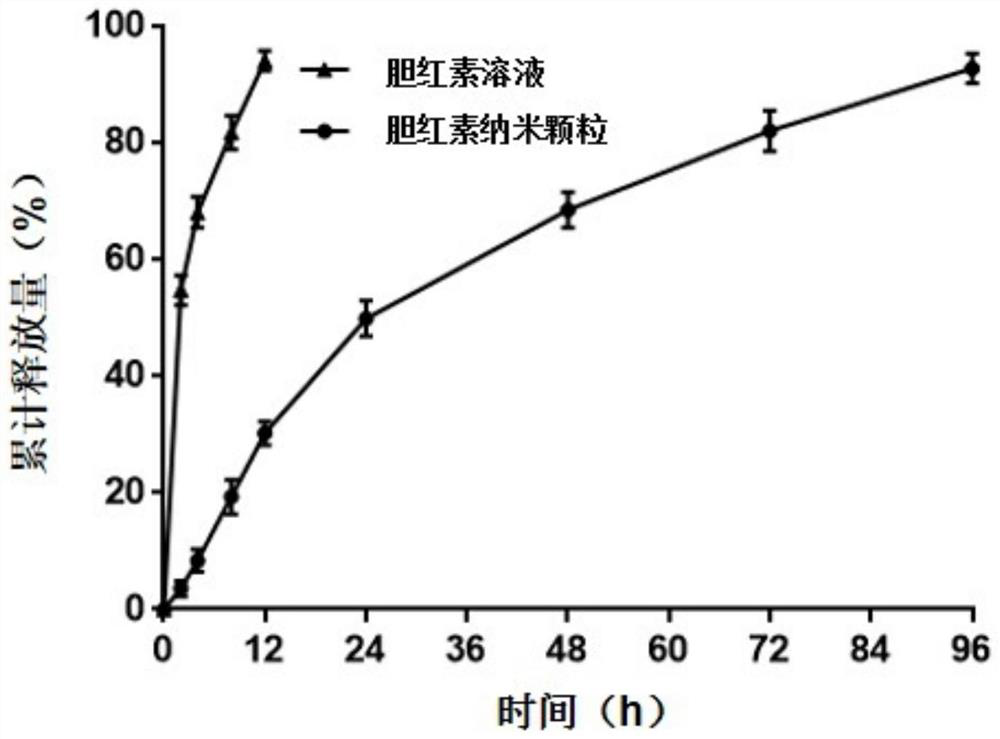
[0062] Example 3 Therapeutic effect of bilirubin nanoparticles on acute pancreatitis
[0063] 3.1 Establishment of acute pancreatitis rat model
[0064] 5-week-old male SD rats were randomly divided into control group, model group and drug intervention group. Before the experiment, the rats were adaptively fed for 1 week before the experiment, and fasted for 12 hours before modeling, and water was not allowed. The mouse was given two intraperitoneal injections of arginine (2.5 mg / ml administration) at intervals of 1 hour, and after the second administration was completed, the amylase increased, which meant that the model was established successfully. After the experiment (that is, 24 hours after the start of modeling), blood was collected from the orbit to collect and separate serum, and stored at -80°C for subsequent measurement of amylase lipase levels. ELISA measured serum inflammatory factors TNF-α, IL-6 levels and The level of the anti-inflammatory factor IL-10; After t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com