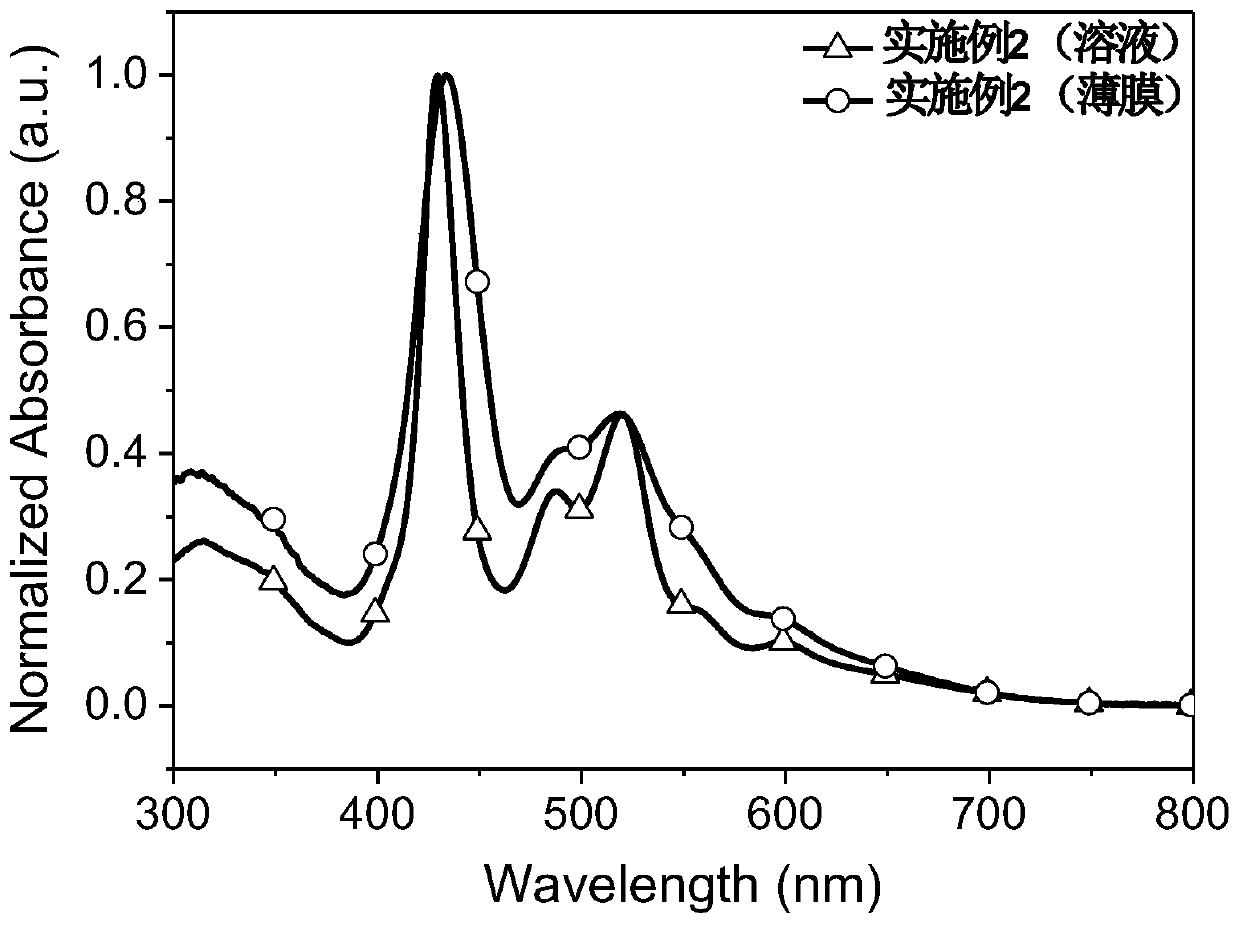
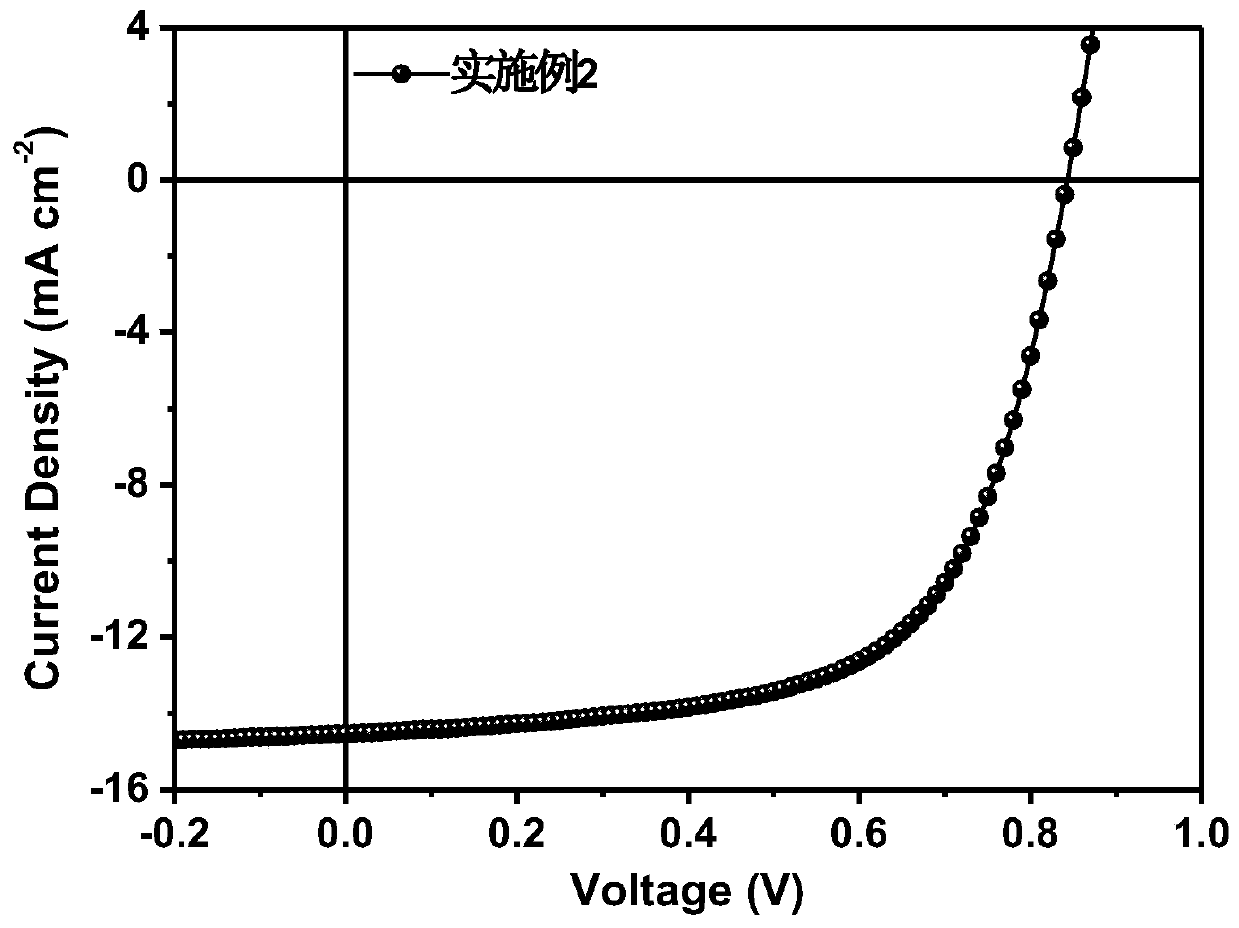
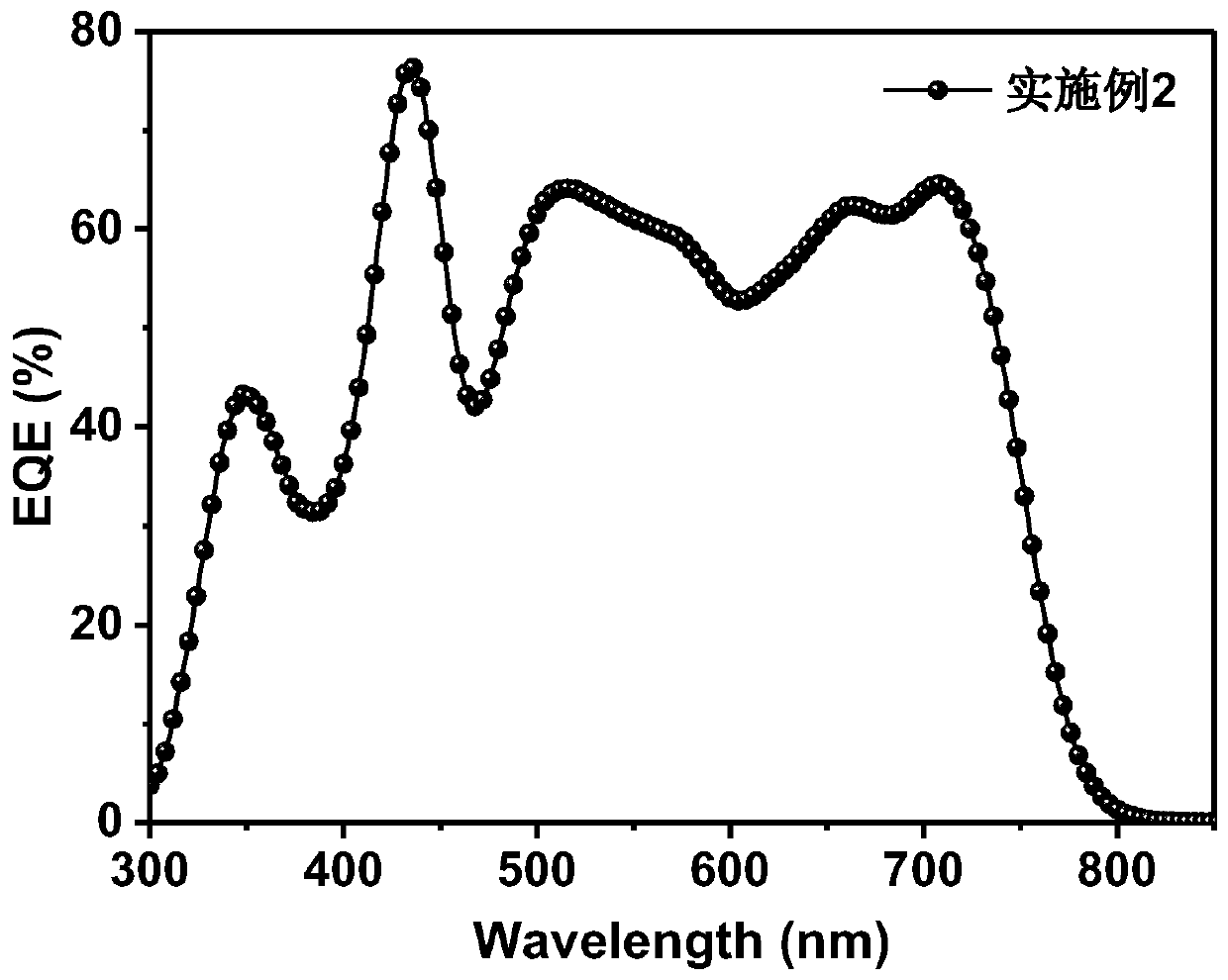
Synthesis method and application of branched porphyrin-perylene diimide small molecule receptor
A perylene diimide and small molecule technology, which is applied in the field of synthesis of branched porphyrin-perylene diimide small molecule receptors, can solve the problems of cumbersome preparation process, low absorption rate and high preparation cost, and achieve High light absorption performance, increase utilization efficiency, and improve the effect of photogenerated current
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] The present invention provides a preparation method of porphyrin-perylene diimide organic small molecule solar cell acceptor material, including the following steps:
[0063] Prepared by the [2+2] synthesis method: Under inert gas atmosphere, organic solvent is used as the reaction medium, and pyrrole is reacted with aldehyde through a catalytic system, and subsequent treatment is used to obtain porphyrin-perylene diimide organic small molecules Acceptor material for solar cells.
[0064] In the embodiment of the present invention, the structural formula of pyrrole is Formula II:
[0065] Formula II:
[0066] The structural formula of the aldehyde is formula III:
[0067] Formula Ⅲ: A-π-CHO;
[0068] Among them: Ar in the structure of pyrrole is the same as Ar in the structure of porphyrin organic small molecule photovoltaic acceptor material; π and A in the structure of aldehyde are the same as those in the structure of porphyrin organic small molecule photovoltaic acceptor mat...
Embodiment 1
[0076] For the synthesis of compound C provided in the embodiment of the present invention, the reaction equation is as follows:
[0077]
[0078] Compound B (514 mg, 0.29 mmol), compound A (77 mg, 0.35 mmol), 37.5 mL of dichloromethane were added to a 100 mL two-necked flask, bubbling under argon for 20 minutes, and then adding trifluoroacetic acid (3.31 mg, 0.029 mmol), stirred overnight at room temperature in the dark. Then add 2,3-dichloro-5,6-dicyanobenzene (77mg, 0.339mmol) and stir for 1 hour, spin off the solvent under reduced pressure and purify it on a chromatographic column. The developing solvent is petroleum ether: three Chloromethane (v:v=1:3), the resulting product was recrystallized in dichloromethane / methanol, and a dark brown solid was obtained as compound C (66.9 mg, 11.7%).
[0079] 1 H NMR (400MHz, Chloroform-d) δ 9.19-8.84 (m, 8H), 8.66 (m, 20H), 8.28 (m, 16H), 7.93-7.69 (m, 10H), 7.64 (m, 16H), 6.26--4.79(m,8H), 2.19m,16H), 2.05-1.67(m,16H), 1.43-1.02(m,128...
Embodiment 2
[0081] For the synthesis of compound D provided in the embodiment of the present invention, the reaction equation is as follows:
[0082]
[0083] Add compound C (61mg, 0.015mmol), zinc acetate (33mg, 0.15mmol), 15mL chloroform into a 100mL two-necked flask, stir at room temperature for 4 hours, spin dry the solvent under reduced pressure, and chromatograph on silica gel. Purify in the plate, the developing agent is petroleum ether: chloroform (v:v=1:3). The resulting product was recrystallized in dichloromethane / methanol to obtain a dark brown solid as compound D (56 mg, 91%).
[0084] 1 H NMR (400MHz, Chloroform-d) δ9.28-8.89(m,8H), 8.86-8.53(m,20H), 8.44-8.16(m,16H), 7.86-7.69(m,10H), 7.70-7.55 (m,16H),5.27-4.83(m,8H),2.51-2.02(m,16H),2.04-1.69(m,16H),1.47-0.98(m,128H),1.02-0.46(m,48H) . 13 C NMR (101MHz, Chloroform-d) δ147.86,146.21,145.15,142.86,142.41,141.40,138.71,137.39,136.01,135.03,134.87,134.83,134.58,133.90,132.49,132.22,132.09,131.96,130.05,129.73,129.33 ,128.56,128...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com