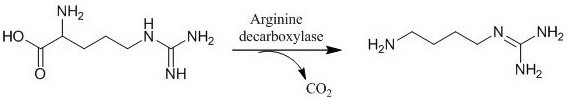
A kind of arginine decarboxylase genetically engineered bacteria and its high-density fermentation culture method
A technology of arginine decarboxylase and genetically engineered bacteria, which is applied in the field of bioengineering, can solve the problem that the enzyme activity and transformation ability of recombinant arginine decarboxylase are different, affect the fermentation production capacity of genetically engineered bacteria, and the expression level of arginine decarboxylase. It can reduce the fermentation cost, improve the utilization rate, and achieve the effect of stable performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] The construction of embodiment 1 engineering bacterium
[0061] Expressing the arginine decarboxylase derived from E.coliMG1655 strain, the base sequence of the arginine decarboxylase gene in the genetically engineered bacteria is shown in SEQ ID NO.1. The construction method of genetically engineered bacteria is as follows:
[0062] Since there is no intron in the adiA gene of E.coliMG1655, the bacterial genome was extracted using a bacterial genome extraction kit. And design primers according to the nucleotide sequence of the target gene. And the restriction enzyme cutting sites SacI and BamHI were respectively added in the forward primer and the reverse primer.
[0063] Forward primer F: 5'-CGAGCTCGAATGCGAAAGTGCGTGTATTG-3', as shown in the nucleotide sequence of SEQ ID NO.3;
[0064] Reverse primer R: 5'-CGGGATCCCGTACTTTCATAATTAACAAC-3', as shown in the nucleotide sequence of SEQ ID NO.4.
[0065] The PCR reaction system and conditions are as follows:
[0066] P...
Embodiment 2
[0076] Embodiment 2: Optimization of fermentation medium and fermentation conditions
[0077] (1) Optimization of fermentation medium
[0078] By screening, it is determined that the composition of the fermentation medium is:
[0079] Glycerin 5-52g / L, peptone 8-45g / L, yeast powder 2-36g / L, Na 2 HPO 4 12H 2 O 6~50g / L, K 2 HPO 4 ·3H 2 O 3-46g / L, potassium dihydrogen phosphate 3-35g / L, sodium chloride 0.5-18g / L, magnesium sulfate 0.5-15g / L, and the balance is water.
[0080] Using eleven factors and two levels L 12 (2 11 ) Orthogonal test method to further optimize the Escherichia coli fermentation medium, and take the enzyme activity of the unit fermentation broth as the response value, determine the optimal medium composition: glycerol 7g / L, peptone 15g / L, yeast powder 5g / L, Na 2 HPO 4 12H 2 O 18g / L, K 2 HPO 4 ·3H 2 O 13g / L, potassium dihydrogen phosphate 5g / L, sodium chloride 1g / L, magnesium sulfate 1g / L.
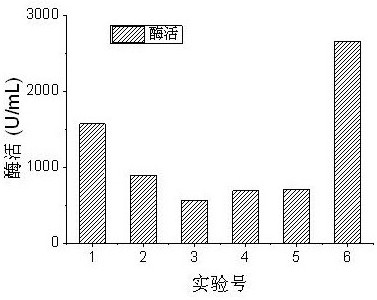
[0081] In order to verify the superiority of this cul...
Embodiment 3
[0089] Embodiment 3: the cultivation of seed liquid
[0090] (1) small test cultivation
[0091] Take the glycerol tube and inoculate it on the Petri dish plate to activate the strains, and cultivate for 24 hours; store the Petri dish plate in a refrigerator at 4°C; use an inoculation loop to dig out a ring of plate seeds under aseptic conditions and inoculate it in the seed medium (50mL / 500mL Erlenmeyer flask). The composition of the medium is: peptone 10g / L, yeast powder 5g / L, NaCl 10g / L, pH natural, sterilized at 121°C and 0.1Mpa pressure for 20min. Then the inoculated seed shake flask was cultured in a constant temperature air bath shaker at 37° C. and 180 rpm for 16 hours to obtain a seed culture solution for small-scale fermentation.
[0092] (2) Pilot test cultivation
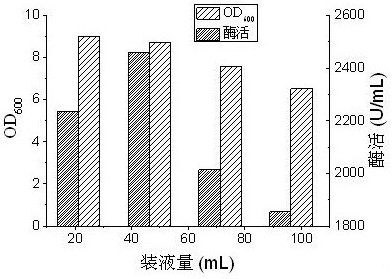
[0093] Take the glycerol tube and inoculate it on the Petri dish plate to activate the strains, and cultivate for 24 hours; store the Petri dish plate in a refrigerator at 4°C; use an inoculation loo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com