Laccase TvLac, and coding gene and application thereof
A technology of laccase and gene, applied in the field of laccase TvLac and its coding gene and application, can solve the problems of limitation and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Cloning of embodiment 1 laccase TvLac coding gene
[0041] The laccase TvLac gene was derived from Trametes versicolor. Specific primers were designed and amplified by PCR. The primer sequences were as follows:
[0042] TvLac-F: 5'-GCTGAAGCTTACGTAGAATTCGCCATTGGGCCCGTCACCGA-3';
[0043] TvLac-R: 5'-AAGGCGAATTAATTCGCGGCCGCGAGGTCGGACGAGTCCA-3'.
[0044] The PCR product was separated by 1% agarose gel electrophoresis, recovered and purified, and its nucleotide sequence was shown as SEQ ID No.4 after analysis.
[0045] Using restriction endonucleases EcoRI and NotI to carry out double enzyme digestion on the product obtained from the PCR amplification gene and the pPIC9 vector, and the digestion products were separated by 1% agarose gel electrophoresis and recovered and purified. T4 DNA ligase was used to ligate the digested vector and the gene fragment and transform the Pichia cloning host to obtain the recombinant Pichia expression plasmid pPIC9-TvLac.
Embodiment 2
[0046] Embodiment 2 Preparation of recombinant laccase TvLac
[0047] The obtained recombinant Pichia expression plasmid pPIC9-TvLac containing laccase TvLac was transformed into Pichia GS115 to obtain recombinant Pichia GS115 / TvLac.
[0048] Take the Pichia pastoris GS115 engineering strain containing the target gene TvLac, inoculate it in 50mL YPD culture medium, and culture it with shaking at 220rpm at 30°C for 48h, then transfer it to 300mL BMGY medium at a ratio of 2%, and culture it with shaking at 220rpm at 30°C for 48h. Replace the BMMY medium (containing 0.2mM CuSO4), and use methanol to induce the expression of exogenous laccase protein. Supplement 0.5% methanol every 24h, a total of 72h induction. The cells were removed by centrifugation at 12000 rpm, the supernatant was concentrated to 5 ml by using a membrane bag, and dialyzed in 10 mM disodium hydrogen phosphate-citric acid buffer (pH 6.5) overnight. Anion column chromatography was used for purification, and th...
Embodiment 3
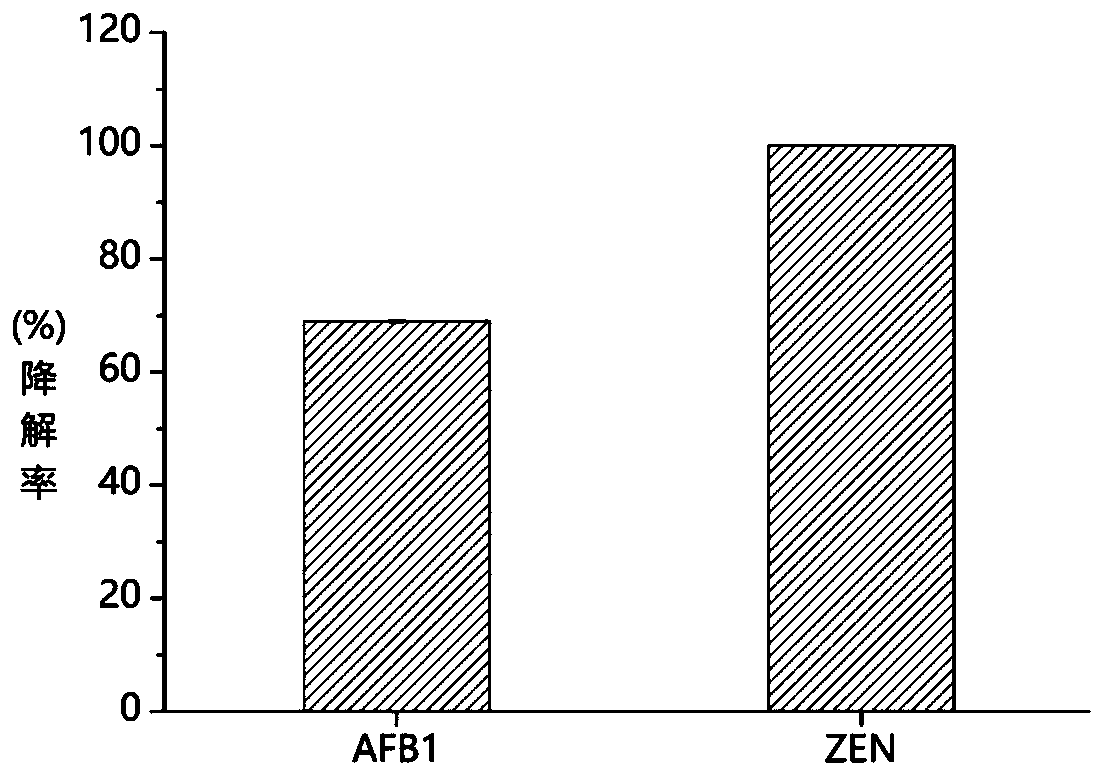
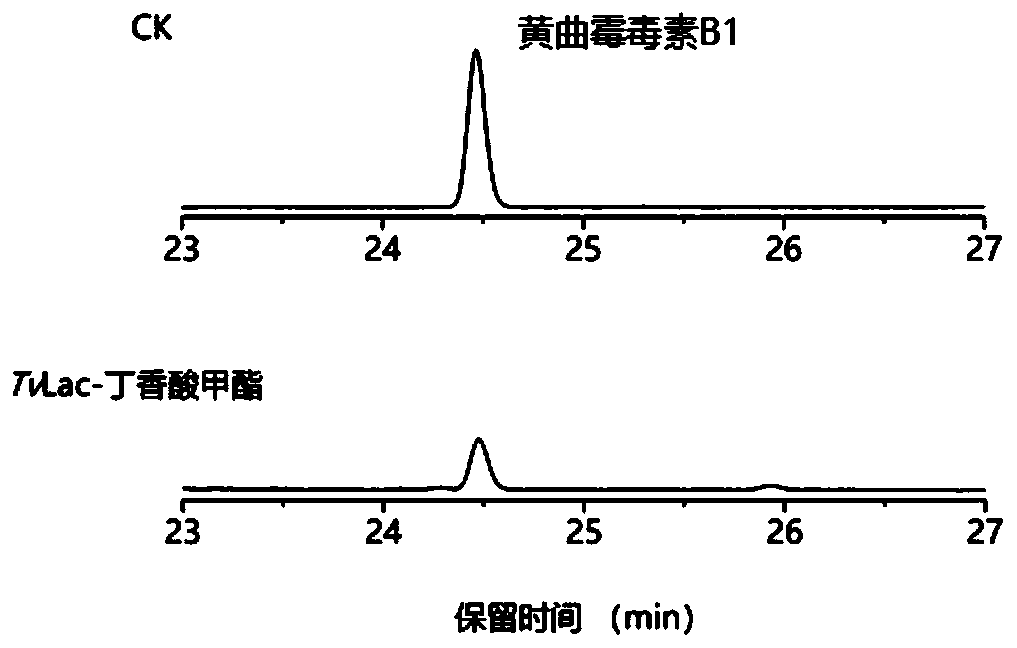
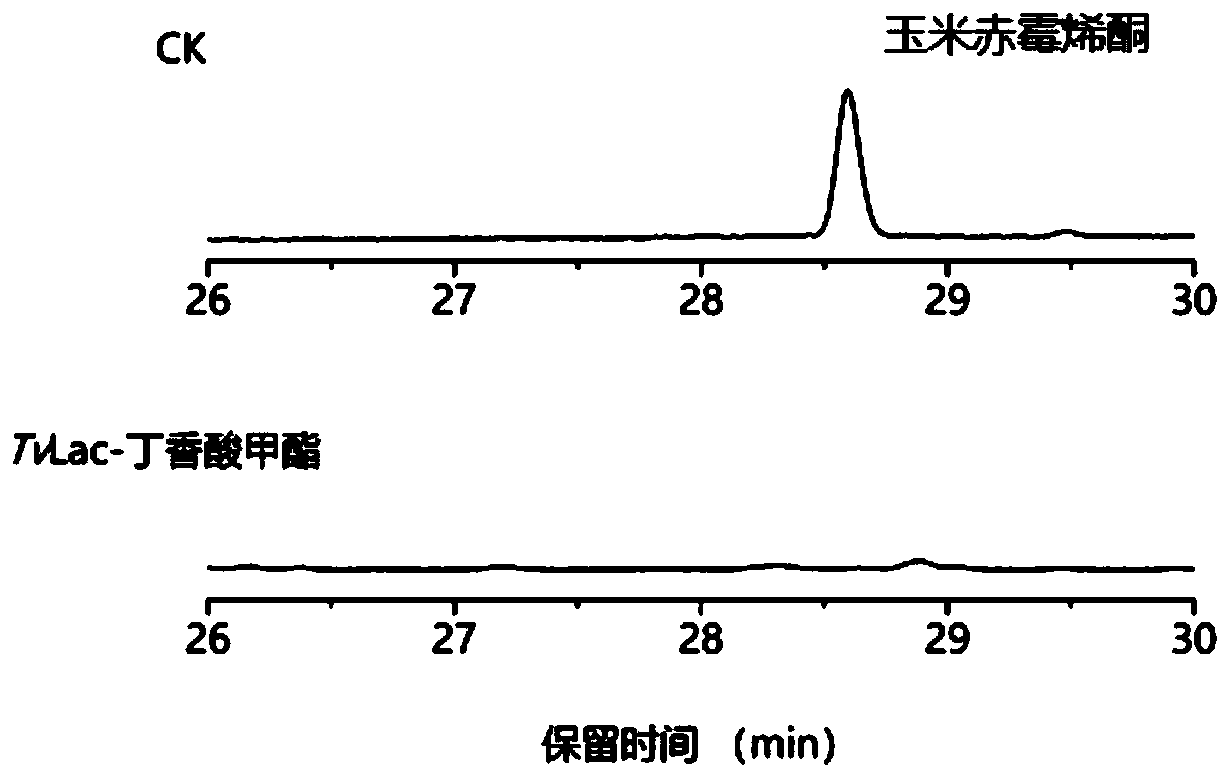
[0049] Example 3 Degradation of aflatoxin B1 by TvLac-methyl syringate mediator system
[0050] Dissolve aflatoxin B1 in dimethyl sulfoxide to prepare a 50mg / L mother solution, as follows: 50mM Tris-HCl (pH 7.0), 20μL aflatoxin B1 solution (50mg / L), 20μL syringic acid Methyl ester (10mM), 20μL TvLac (300U / L). The system without adding laccase TvLac was used as a control, and the reaction system was set in 3 repetitions. The reaction was carried out at 30° C., and three times the volume of acetonitrile was added to terminate the reaction after 10 hours. The degradation rate of aflatoxin B1 was analyzed by high performance liquid chromatography (HPLC). The liquid chromatography is a Shimadzu Nexera UHPLC high-performance liquid chromatography analysis system, and the chromatographic separation column is Zorbax SB-C18 (4.6×250mm, 5μm), mobile phase A (water with 0.06% TFA), mobile phase B (water with 0.05% TFA Acetonitrile); Gradient elution condition 0% B elution 4 minutes, 0%...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com