Pharmaceutical composition containing dabigatran etexilate and preparation method thereof
一种达比加群酯、达比加群酯甲磺酸盐的技术,应用在含达比加群酯的药物组合物及其制备领域,能够解决副作用、治疗指数低等问题,达到促进吸收、缓解出血、降低吸收变异性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
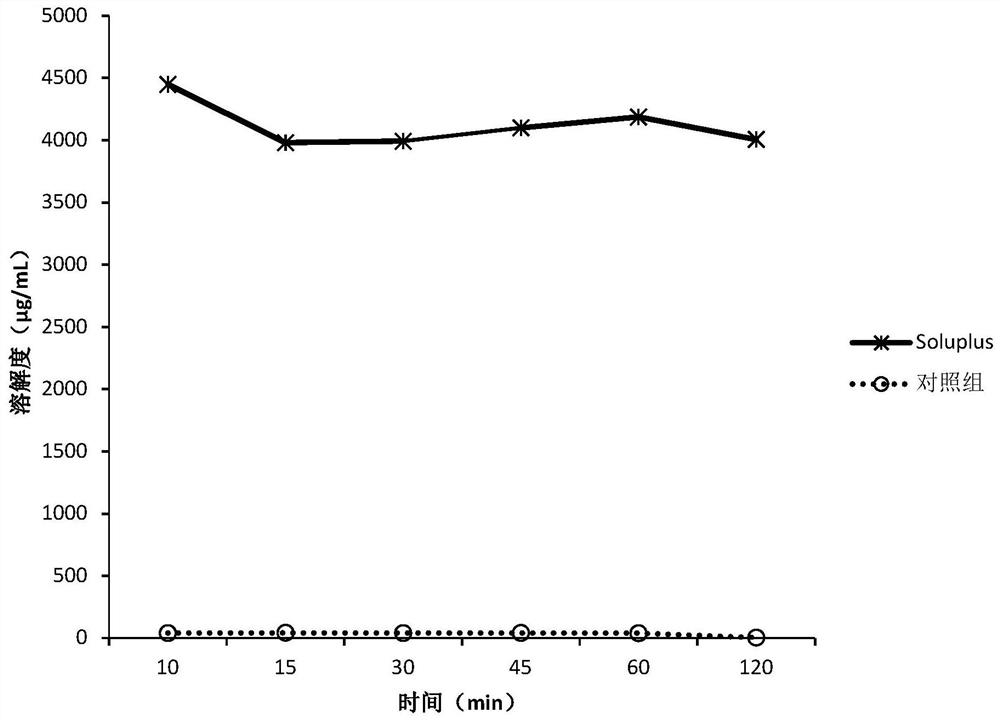
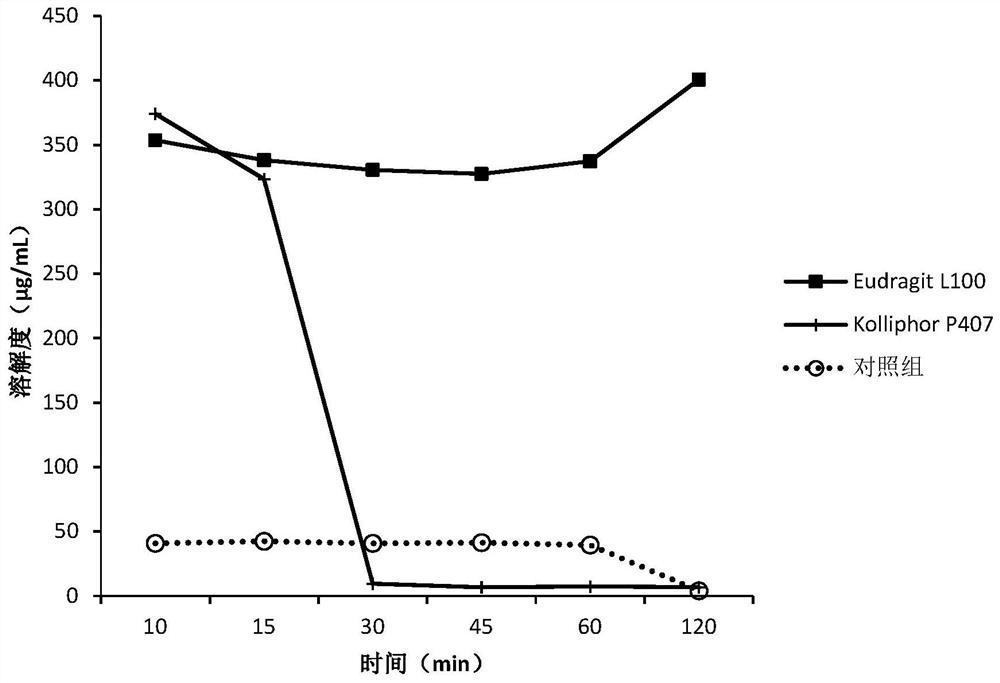
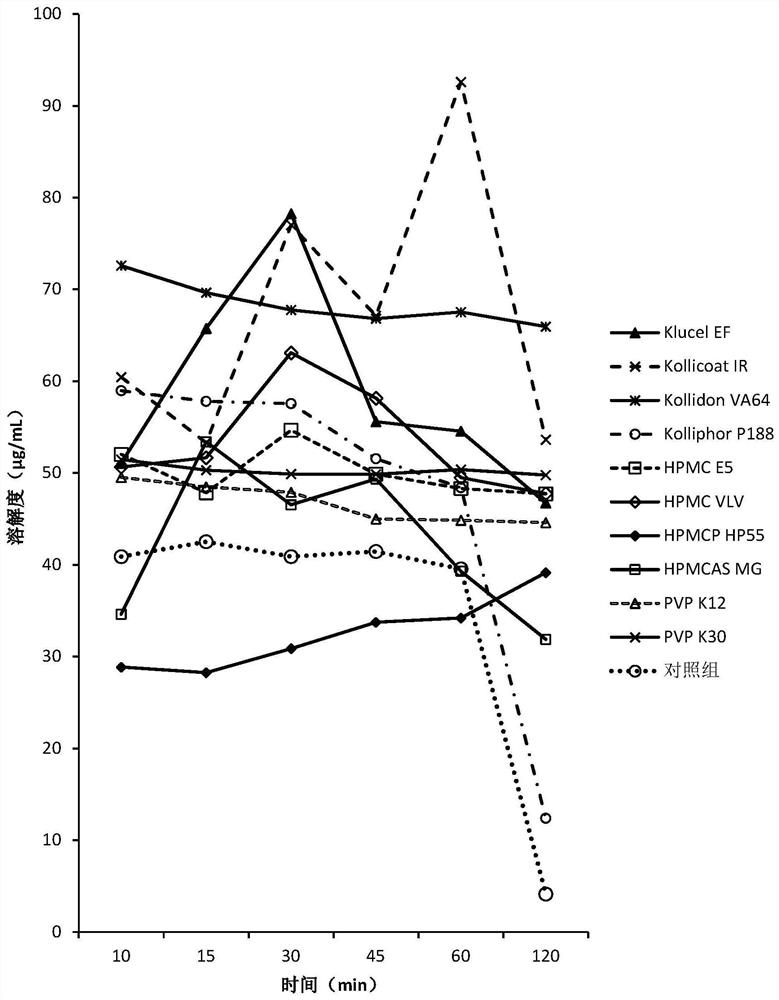
[0151] The screening experiment carried out in Example 1 aims at finding a polymer capable of inhibiting the precipitation of DEM at neutral pH, and the following polymers are intended to be used in the screening experiment.
[0152] Eudragit L100Klucel EF Kollicoat IR Kollidon VA64KolliphorP188Kolliphor P407HPMC E5HPMC VLV HPMCP HP55HPMCAS MG PVP K12PVP K30Soluplus
[0153] Screening experiments include the following steps:
[0154] 1. Prepare DEM stock solution at 50 mg / mL drug concentration in 0.1 N HCl.
[0155] 2. Put 40 mg of the above polymer into a 5 mL vial, add 3.6 mL of sodium phosphate buffer solution with pH 6.8 to dissolve the polymer, and obtain a polymer solution.
[0156] 3. Add 0.4 mL of the stock solution from step 1 to the polymer solution from step 2, while adding 0.4 mL of the stock solution to pH 6.8 phosphate buffer without polymer as a control.
[0157] 4. Shake the vial at 250 rpm under ambient room temperature conditions.
[0158] 5. Take 0.4 mL a...
Embodiment 2
[0167] Embodiment 2 is a micro-dissolution experiment, the purpose is to evaluate the dissolution behavior of DEM solid dispersion film (SDF), the following table is the composition of the SDF to be evaluated in this micro-dissolution experiment, and each percentage refers to the mass percentage.
[0168]
[0169] In this example, DEM SDF containing 3mg of DEM was prepared in a 20mL glass bottle by solvent evaporation method, and then the dissolution medium was added to carry out the dissolution test at 37°C. The specific experimental steps are as follows:
[0170] 1. Prepare a DEM stock solution with a drug concentration of 100 mg / mL in an ethanol solution with a volume fraction of 95%.
[0171] 2. Prepare a polymer stock solution at a polymer concentration of 100 mg / mL in 95% ethanol by volume.
[0172] 3. According to the volume shown in the table below, add the stock solution in step 1 and step 2 into a 20mL vial and mix thoroughly to obtain a mixed solution;
[0173] ...
Embodiment 3
[0190] Embodiment 3 is a micro-dissolution experiment, the purpose is to evaluate the dissolution behavior of DEM solid dispersion film (SDF) in pH4.5 sodium acetate buffer and pH6.8 sodium phosphate buffer, and the specific experimental steps are the same as in embodiment 2. The specific experimental steps are as follows:
[0191] 1. Prepare DEM stock solution at 100 mg / mL drug concentration in 95% ethanol solution.
[0192] 2. Prepare a polymer stock solution at a polymer concentration of 100 mg / mL in 95% ethanol solution.
[0193] 3. According to the volume shown in the table below, add the stock solution in step 1 and step 2 into a 20mL vial and mix thoroughly to obtain a mixed solution;
[0194] Among them, DEM F is that the solution in step 1 is added to the ethanol solution with a volume fraction of 95% and fully mixed to obtain a mixed solution; DEM P does not do any treatment in this step; the numbered DEM F and DEM P are controls Group.
[0195]
[0196] 4. Add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com