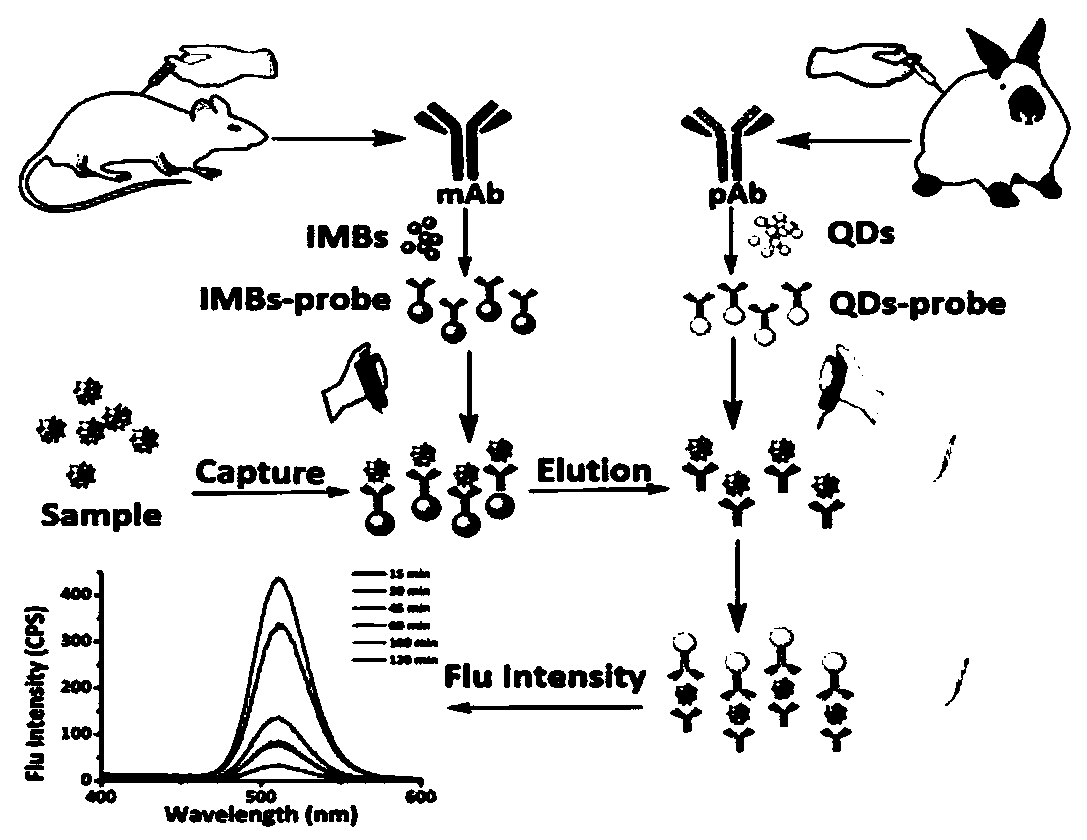
Rapid detection method based on magnetic separation and quantum dot labeling of Helicobacter pylori, and kit
A Helicobacter pylori, anti-Helicobacter pylori technology, applied in the direction of measuring devices, analysis materials, instruments, etc., can solve the problems of long pretreatment time, complex stool sample matrix, and can not meet the requirements of stool antigen detection, and achieve the detection method The effect of simplicity, low detection cost and high practical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
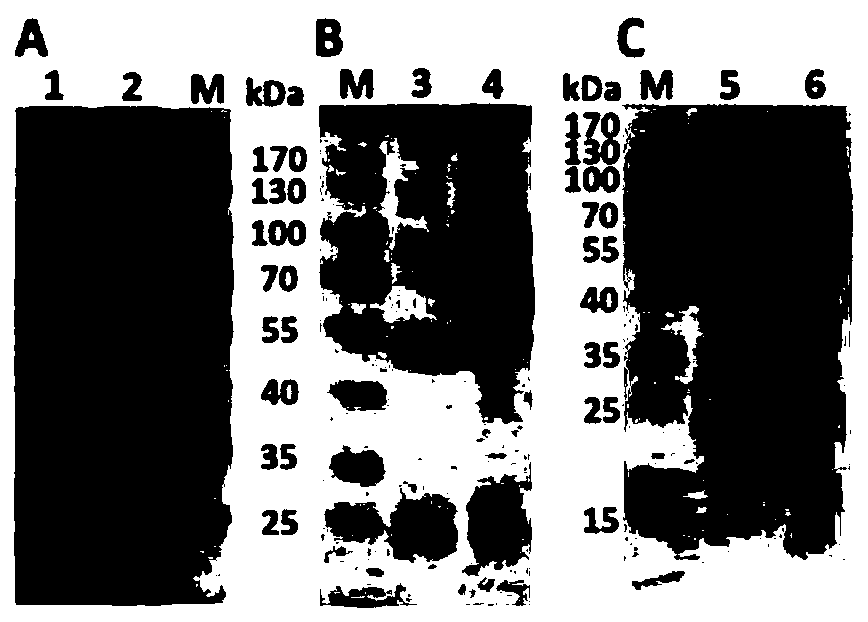
[0064] Example 1 Preparation of Rabbit Anti-Helicobacter Pylori Outer Membrane Protein Polyclonal Antibody
[0065] 1. Preparation of rabbit anti-Helicobacter pylori outer membrane protein (OMPs) protein antigen
[0066] (1) Experimental method
[0067] 1. Culture of Helicobacter pylori (H.P)
[0068] H.P is a spiral, curved or straight unmycobacterium, 0.3~1.0×1.5~5μm, obtuse at the top, Gram negative, microaerophilic, and moves rapidly in a spiral form by 4 to 8 flagella at both ends. Use Columbia blood agar medium (add 5-10% of defibrated sheep blood) to culture colonies without obvious pigment, translucent, and small colonies in the form of needle points, put them into the incubator, adjust the culture conditions, and the gas conditions are 5% O 2 , 10% CO 2 and 85%N 2 (or H 2 ) in a gas environment with a temperature of 37° C. and a high-humidity environment for about three days to collect bacteria.
[0069] 2. Extraction of OMPs protein
[0070] The collected wet ...
Embodiment 2
[0078] Example 2 Preparation of Monoclonal Antibody Using the Urease B Peptide of Helicobacter pylori
[0079] 1. Experimental method
[0080] For the specific preparation process, refer to the method of the patent "Hybridoma Cell Line and Glycocholic Acid Monoclonal Antibody and Detection Kit Based on Hybridoma Cell Line" with application number 201810229091.4, using "Identification of an Antigenic Epitope in Helicobacter pylori Urease That Induces Neutralizing Antibody Production The monoclonal antibody was prepared from the urease B peptide CHHLDKSIKEDVQFADSRI of Helicobacter pylori provided in ".
[0081] The specific method is as follows:
[0082] (1) Animal immunity
[0083] The immunogen is hemocyanin coupled with peptide CHHLDKSIKEDVQFADSRI, which is directly dissolved in PBS to make a concentration of 1 mg / mL. Take 0.5mL of the above solution and emulsify it with an equal volume of complete Freund's adjuvant, inject it into Balb / C mice (5 6-week-old Balb / C female m...
Embodiment 3
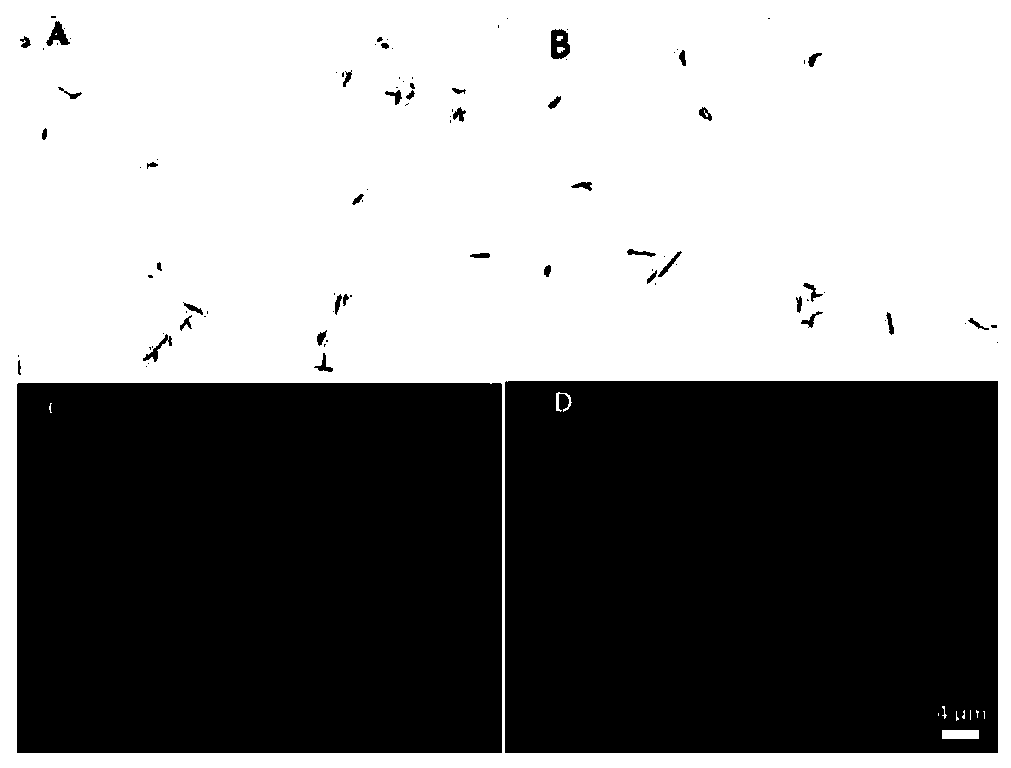
[0101] Indirect immunofluorescence of embodiment 3pAb binding to bacteria
[0102] 1. Experimental method
[0103] (1) Fix: transfer the bacteria to a 1.5mL centrifuge tube (tip), centrifuge to remove the supernatant, add 500μL of ice-cold 4% paraformaldehyde, incubate at room temperature for 20min, and store in PBS at 4℃ for 1 week after fixation ;
[0104] (2) Permeabilization: Centrifuge to remove the supernatant, add 250 μL of permeabilization solution, and incubate at 4°C for 10 minutes;
[0105] (3) Glycine rinsing: centrifuge, absorb the permeate, rinse 3 times with glycine solution at room temperature;
[0106] (4) Blocking: After centrifuging and removing the glycine solution, add 250 μL of blocking solution and incubate at room temperature for 1.5 h;
[0107] (5) Primary antibody pAb incubation: centrifuge and remove the blocking solution, add 250 μL of the pAb of Example 1, and incubate at room temperature for 4 hours to overnight;
[0108] (6) Wash with PBST 3 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com