Technology for regulating and controlling Jak-Stat pathway to differentiate, dedifferentiate and rejuvenate cells, and application of technology
A cell differentiation and de-differentiation technology, applied in animal cells, cell culture active agents, genetically modified cells, etc., can solve the problems of tumorigenesis, genetic variation, unfavorable clinical application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
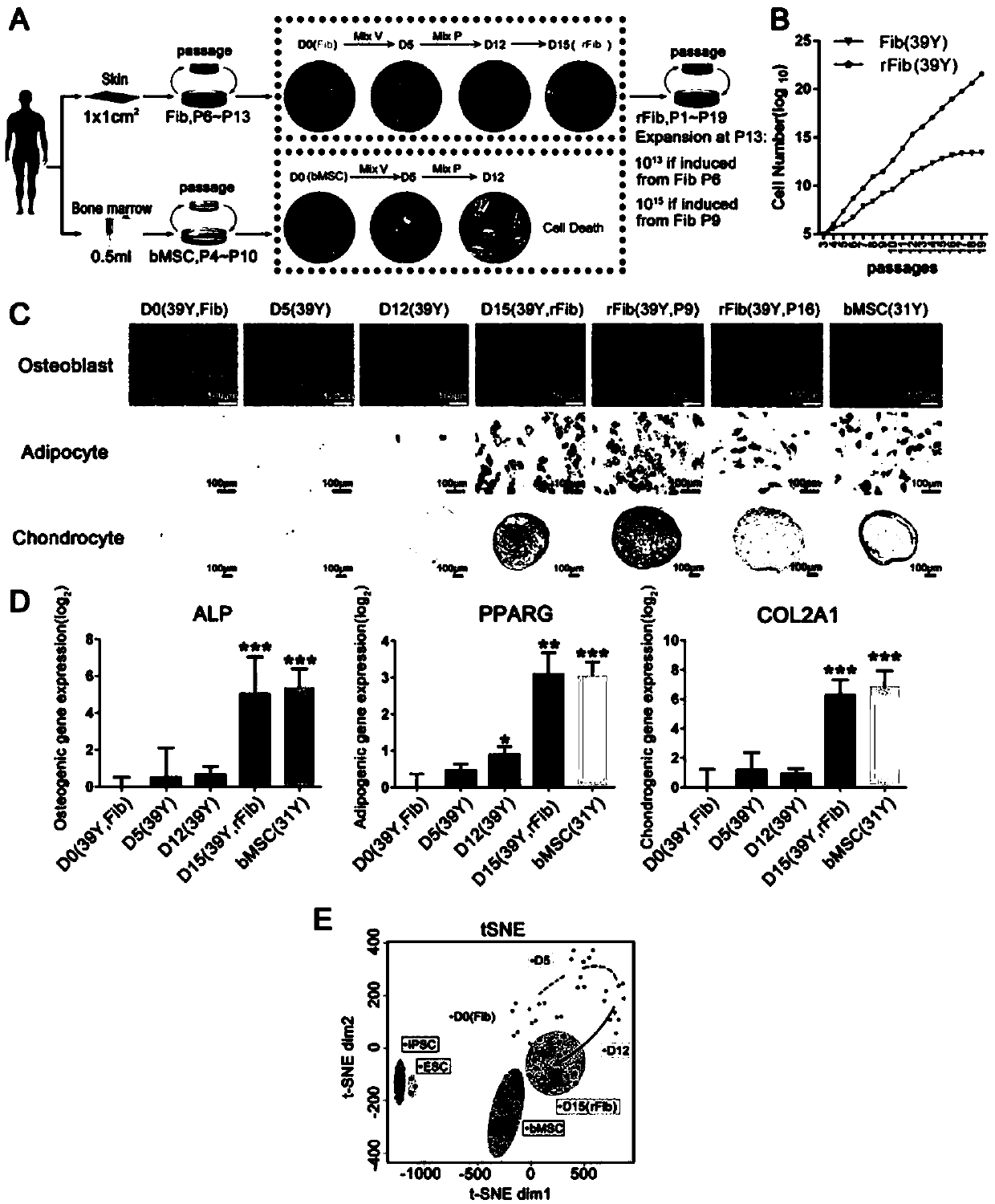
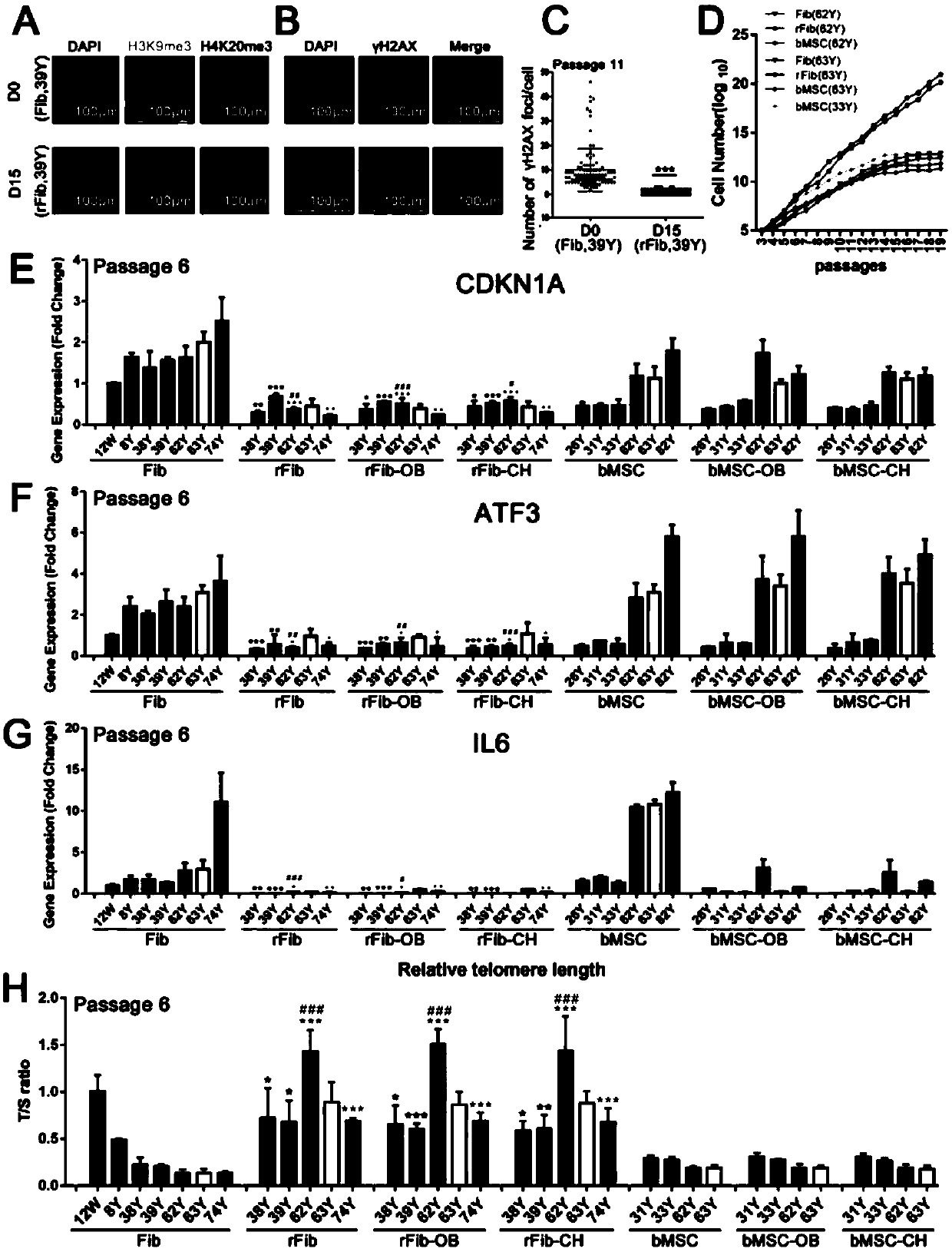
[0101] Example 1, the acquisition and identification of repairing skin fibroblasts
[0102] 1. Human skin fibroblasts were inoculated into 6-well plates and cultured in skin fibroblast culture medium for 24 hours;
[0103] 2. Replace the cell culture medium with the rFib induction medium containing the small molecule cocktail combination Mix V, and change the medium every 2 days;
[0104] 3. After culturing for 5 days with the rFib induction medium containing the small molecule cocktail combination Mix V, replace the culture medium with the rFib induction medium containing the small molecule cocktail combination Mix P, and change the medium once every 2 days;
[0105] 4. After culturing for 7 days with the rFib induction medium containing the small molecule cocktail combination Mix P, the medium was replaced with HG-DMEM supplemented with 10% FBS, 10ng / mL bFGF, 100ng / mL PDGF-AB and 10ng / mL BMP4 ; Or add 10% FBS to HG-DMEM; Or use rFib medium for culture; Cells are treated for...
Embodiment 2
[0152] Example 2 rFib cells have the ability to repair bone defects
[0153] Under the approval of the ethics committee, NOD / SCID mice aged 8 to 10 weeks and weighing 20 to 24 g were used to create a femoral defect model, and 5 animals were used in each group. The modeling method was as follows: under pentobarbital sodium anesthesia, cut The skin and subcutaneous tissue were opened, and a sufficient mid-femur was exposed by blunt dissection between the rectus femoris and semitendinosus. The operation was performed at the proximal junction of the center of the right femur. A continuous bone defect of 4mm×1mm was constructed surgically. Skin fibroblasts (Fib), bone marrow mesenchymal stem cells (bMSC) and rFib were stained with Hoechst 33342 (Thermo, NucBlue live cell), then mixed with Matrigel and transplanted into the defect site with 5×105 cells / mouse.
[0154] Twenty-eight days after transplantation, the mice were sacrificed by injecting a lethal dose of sodium pentobarbit...
Embodiment 3
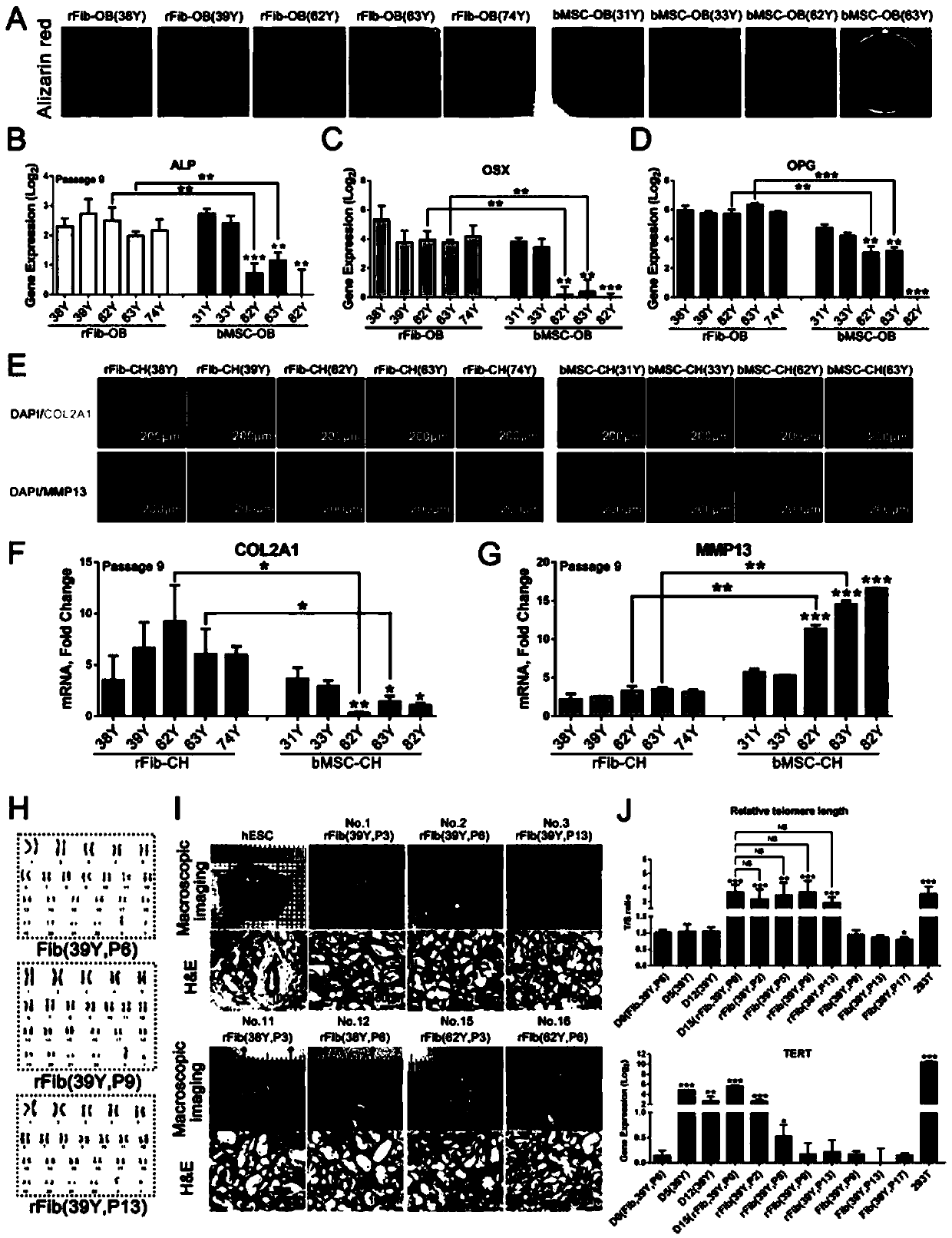
[0161] Example 3, rFib cells have the ability to repair cartilage defects
[0162] Articular cartilage defect model and cell transplantation
[0163] NOD / SCID mice weighing 20-24 g at the age of 8 to 10 weeks were used. A modified articular cartilage model was used to evaluate the efficacy of rFib (Cheng et al., 2014). Articular cartilage defects (1.5 mm x 1 mm) were constructed in the trochlear groove of the distal femur with a biopsy punch. cells ((2.5×10 5 Each in 35 μl Matrigel) was labeled with Hoechst 33342 and implanted into the defect site. Matrigel implants without cells were used as controls.
[0164] Figure 7 .In vivo cartilage repair experiment
[0165] A-B. Safranin Fast Green staining of cartilage tissue samples and 10 μm sections. In safranin fast green staining, red is cartilage and green is bone tissue. Both young rFib (39 years old) and bMSCs (31 years old) can repair cartilage defects. Although old bMSCs (62 years old) can generate new tissue, they ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com