Preparation method and application of non-noble metal monoatomic catalyst
A non-precious metal and catalyst technology, applied in the fields of chemistry, chemical engineering and material science, to achieve the effects of low preparation cost, improved atom utilization and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
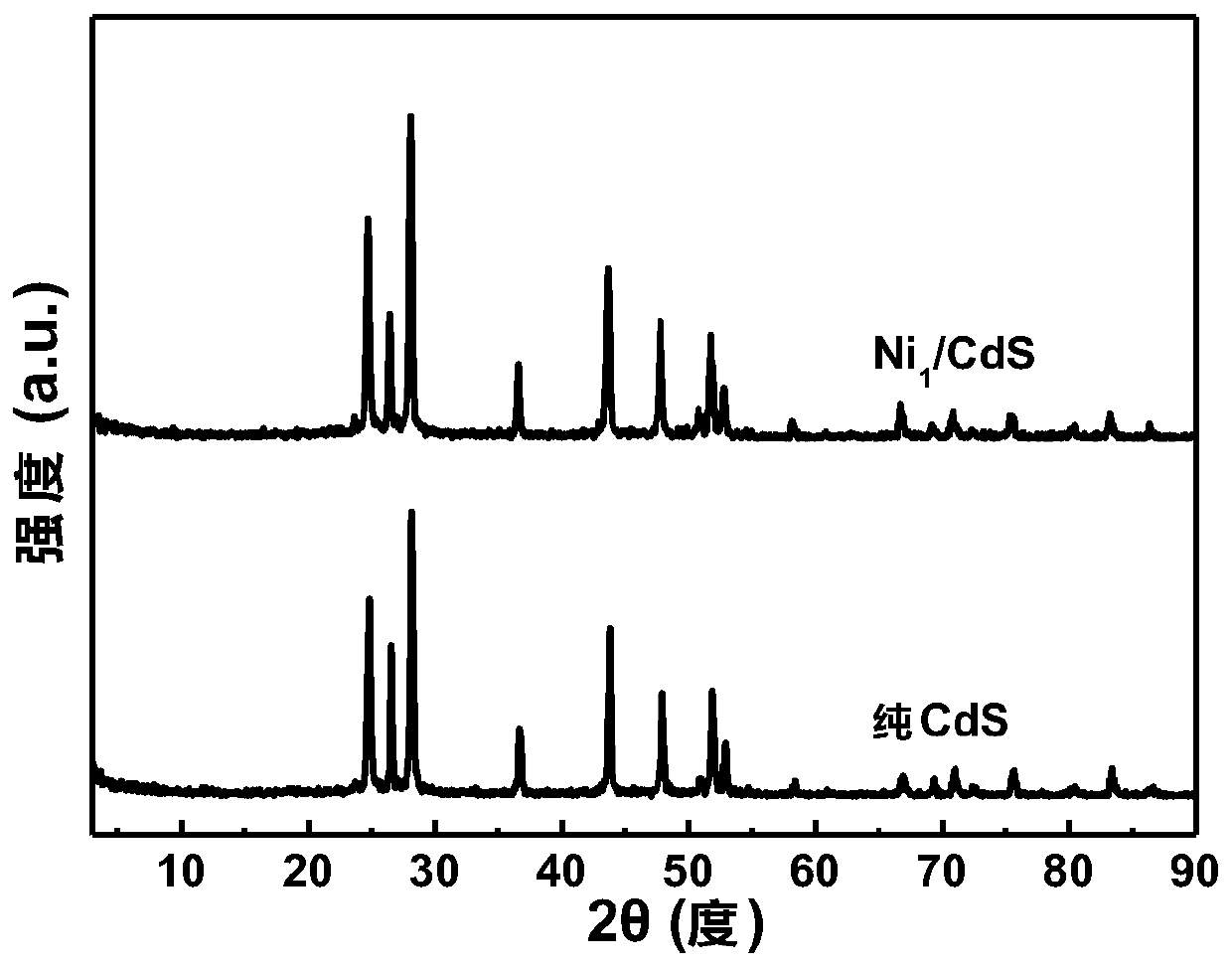
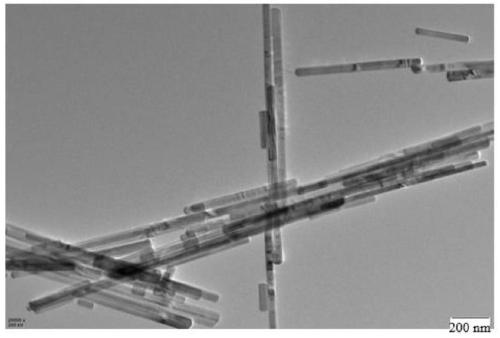
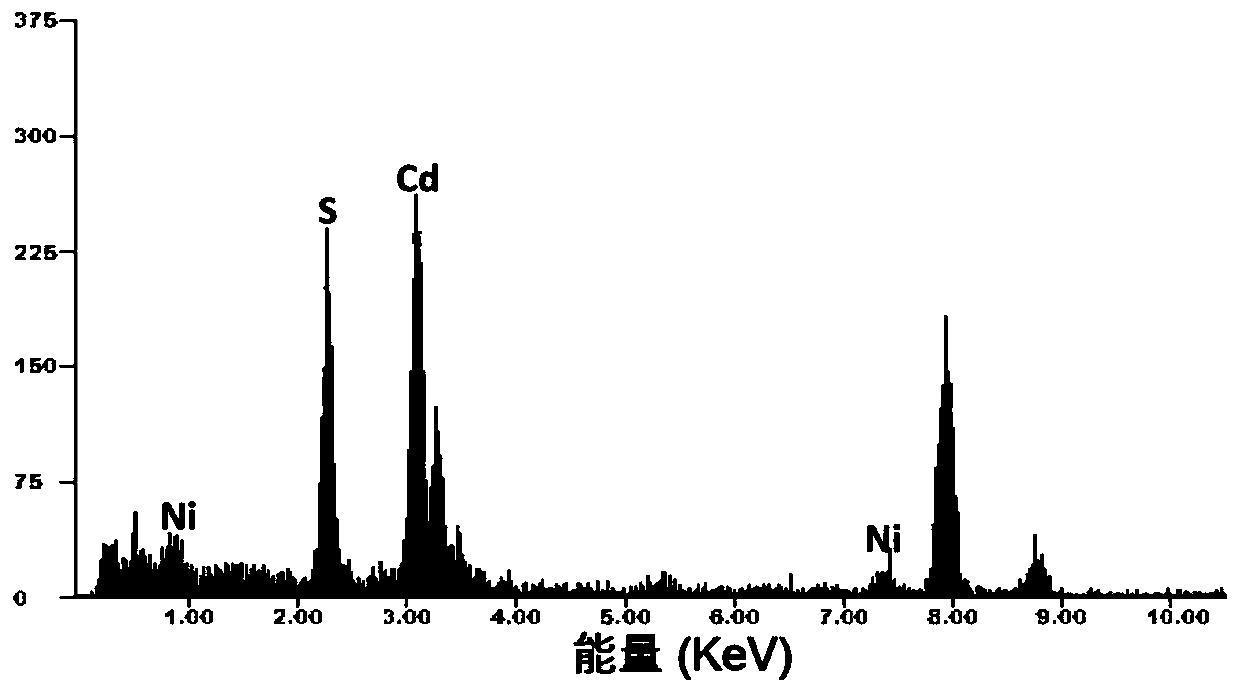
Image
Examples
Embodiment 1
[0069] Embodiment 1: Preparation of nickel single atom / cadmium sulfide nanorod composite catalyst
[0070] Prepare nickel single atom / cadmium sulfide composite catalyst according to the following method
[0071] (1) Take 20.25mmol of cadmium chloride 2.5 hydrate, 40.75mmol of thiourea and 60mL of ethylenediamine in a 100mL high-pressure reactor, and place the reactor in a 160°C oven for hydrothermal treatment for 48 hours. After the reaction, remove the reactor Put it under natural conditions and drop it to room temperature, filter to obtain a yellow solid, wash with deionized water 10 times, and wash with ethanol twice, and place the obtained solid in an oven at 60°C for 12 hours to obtain a yellow solid that is cadmium sulfide nanorods;
[0072] (2) Take 50mg of cadmium sulfide nanorods and place them in a 25mL single-necked flask, then add 1mL of nickel acetate aqueous solution (12.5mg / mL), 1mL of thiourea aqueous solution (38mg / mL) and 8mL of deionized water, ultrasonicall...
Embodiment 2
[0075] Example 2: Catalytic activity of nickel single atom / cadmium sulfide nanorod composite catalyst
[0076] Take 2 mg of cadmium sulfide obtained in step (1) of Example 1 and place it in a 100 mL photocatalytic reactor, then add 10 mL of lactic acid and 40 mL of water. Sonicate for 30s, use nitrogen degassing for 1h to remove oxygen in the system, place the round bottom flask under 300W xenon light (equipped with a 420nm cut-off filter) and irradiate, after the reaction is over, use thermal conductivity-gas chromatography to detect the generated in the reaction Hydrogen, the hydrogen production rate is 4.8mmol·g after 6 hours of reaction -1 h -1 .
[0077] Ni obtained in Example 1 1 2mg / CdS NRs composite catalyst was placed in a 100mL photocatalytic reactor, followed by adding 10mL lactic acid and 40mL deionized water. Sonicate for 30s, use nitrogen degassing for 1h to remove oxygen in the system, place the round bottom flask under 300W xenon light (equipped with a 420n...
Embodiment 3
[0082] (1) Take 20.25mmol of cadmium chloride 2.5 hydrate, 40.75mmol of thiourea and 60mL of ethylenediamine in a 100mL high-pressure reactor, and place the reactor in a 160°C oven for hydrothermal treatment for 48 hours. After the reaction, remove the reactor Put it under natural conditions and drop it to room temperature, filter to obtain a yellow solid, wash with deionized water 10 times, and wash with ethanol twice, and place the obtained solid in an oven at 60°C for 12 hours to obtain a yellow solid that is cadmium sulfide nanorods;
[0083] (2) Take 50mg of cadmium sulfide nanorods and place them in a 25mL single-necked flask, then add 1mL of nickel acetate aqueous solution (12.5mg / mL), 1mL of thiourea aqueous solution (38mg / mL), and 8mL of deionized water, ultrasonically disperse for 1min, and then Use nitrogen degassing for 40 minutes to remove oxygen in the reaction system;
[0084] (3) After the degassing is completed, place the round bottom flask under a 300W xenon ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com