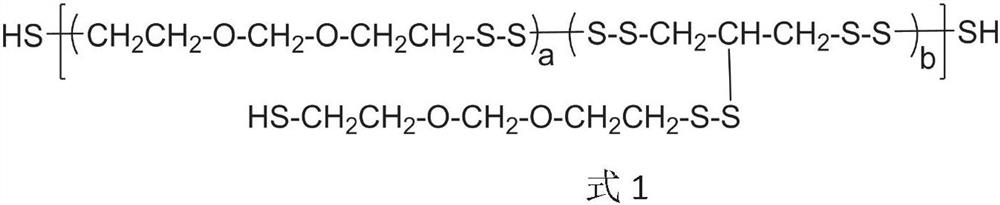Epoxy terminated polysulfide rubber and its preparation and application
A polysulfide rubber and epoxy-terminated technology, used in adhesives, other chemical processes, chemical instruments and methods, etc., can solve the doubt of conversion rate and reactivity, difficulty in removing olefinic compounds, poor anti-aging performance, etc. problems, to achieve the effects of easy control of the reaction, fast reaction rate and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
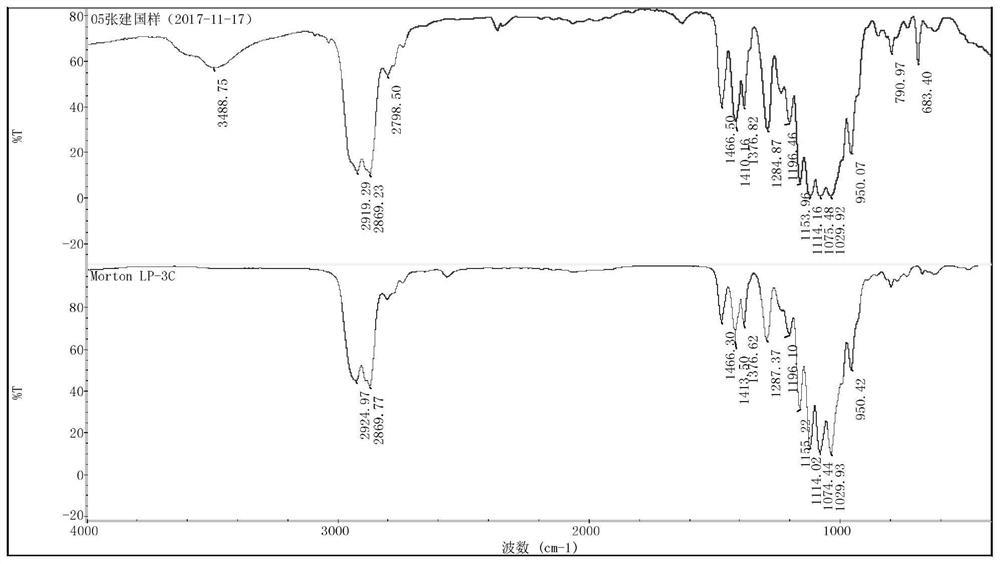
[0061] The first step is to put 100g of JLY-121 polysulfide rubber (average molecular weight of 1000, viscosity of 8pa.S, mercapto group content of 6.50%) and 400g of toluene into a 1L clean three-necked flask, respectively, and remove the material by azeotropic dehydration. After the saturated water in the solution was cooled to below 80°C, under nitrogen protection and stirring, at the temperature of the reaction solution at 10-80°C, 1.0mol / L of n-butyllithium 206mL was continuously added dropwise within 20min to the reaction in the flask After 40 minutes of reaction, 0.6 mL of ethanol was inhaled with a syringe and injected into the reaction bottle to terminate the reaction for 20 minutes.
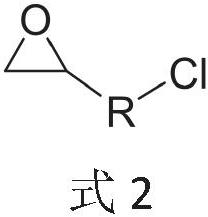
[0062] The second step is to add 18.5 g of epichlorohydrin to the reaction solution of the first step, stir the reaction at 50-80 ° C for 60 minutes, then add 20 mL of deionized water to the reaction solution and stir for 15 minutes, and then the water phase is removed statically. Final...
Embodiment 2
[0065] The relevant process conditions in Example 1 were kept unchanged.
[0066] The polysulfide rubber selected in the first step is JLY-1225 type (average molecular weight is 2500, viscosity 17pa.S, mercapto group mass content 2.62%) 100g, toluene 100g, n-butyllithium used is 87mL, the reaction time 30min, add 0.5mL of terminating agent ethanol.
[0067] 8.08 g of epichlorohydrin was added in the second step reaction, the reaction time was 120 min, after the reaction was completed, 30 mL of deionized water was used to stir for 15 min, the water phase was statically separated, and the solvent and other low-boiling organic impurities were removed by distillation under reduced pressure. . The viscosity of the amber viscous liquid measured by analysis is 18.5pa.S, and the epoxy value is 0.076mol / 100g.
Embodiment 3
[0069] The relevant process conditions in Example 1 were kept unchanged.
[0070] The polysulfide rubber selected in the first step is JLY-124 type (average molecular weight is 4000, viscosity 70pa.S, mercapto group mass content 1.64%) 100g, toluene 300g, n-butyllithium used is 54mL, the reaction time 25min, 0.4mL of terminating agent ethanol was added.
[0071] 4.8 g of epichlorohydrin was added in the second step reaction, the reaction time was 80 min, after the reaction was completed, 40 mL of deionized water was used to stir for 15 min, the water phase was statically separated, and the solvent and other low-boiling organic compounds were removed by distillation under reduced pressure. impurities. The viscosity of the amber viscous liquid measured by analysis is 72.1pa.S, and the epoxy value is 0.048mol / 100g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| epoxy value | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com