Biosynthesis method of nicotinamide adenine dinucleotide compound
A nicotinamide adenine and dinucleotide technology, applied in the field of biosynthesis of nicotinamide adenine dinucleotide compounds, can solve the problems of expensive consumption, limited concentration, high production cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0159] Embodiment 1, establishment of synthetic route
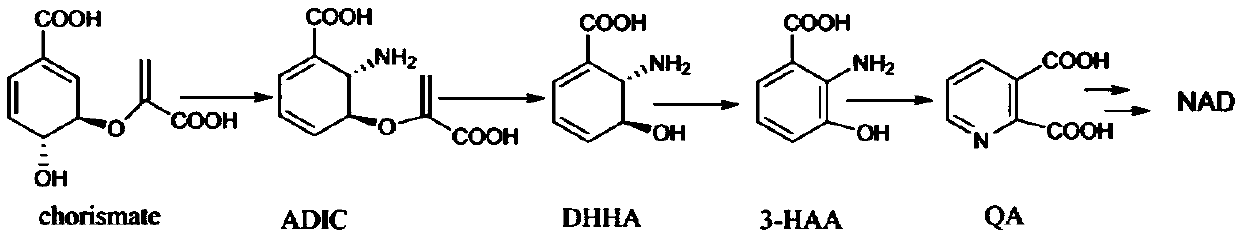
[0160] Through a large number of investigations and studies, a new NAD synthesis route was established ( figure 1 ).
[0161] With chorismic acid as the starting point, aminodeoxyisochorismic acid (ADIC) is generated through transamination rearrangement under the action of PhzD protein, and aminodeoxyisochorismic acid is catalyzed by PhzE protein to remove the pyruvate part to generate 2,3-di Hydrogen-3-hydroxyanthranilic acid (DHHA), catalyzed by a specific enzyme (DHHA-2,3-dehydrogenase) from 2,3-dihydro-3-hydroxyanthranilic acid to 3-hydroxyanthranilic acid Formic acid (3-HAA), under the action of NabC protein, 3-hydroxyanthranilic acid undergoes an oxidative ring-opening rearrangement reaction to generate quinolinic acid (QA), and quinolinic acid in vivo is under the action of quinolinic acid phosphotransferase React with pyrophosphate ribose to generate nicotinic acid mononucleotide, and then enter the NAD salvage ...
Embodiment 2
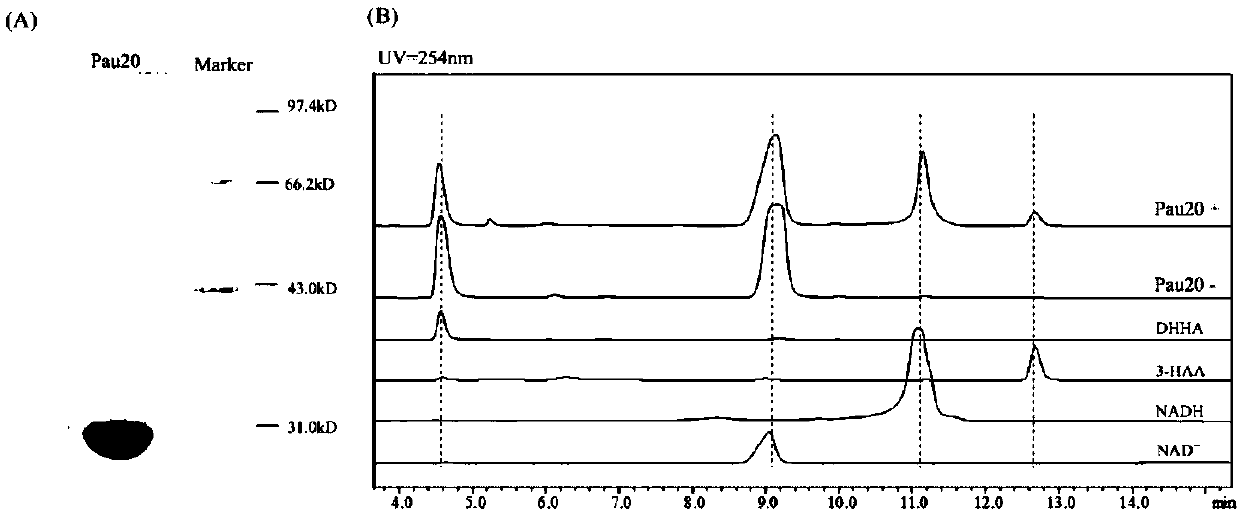
[0165] Embodiment 2, the identification of DHHA-2,3-dehydrogenase
[0166] 1. Identification of Pau20
[0167] 1. An enzyme that may have DHHA-2,3-dehydrogenase activity was screened out from a large number of candidate enzymes, and it was named Pau20. Its coding gene is shown in sequence 1 of the sequence listing, and its protein sequence is shown in sequence listing Sequence 2 is shown.
[0168] 2. Preparation and purification of Pau20
[0169] (1) The small fragment between the NdeI and BamH I restriction sites of the pET28a plasmid (Novagen) is replaced by the double-stranded DNA molecule shown in Sequence 1 of the sequence listing to obtain the recombinant plasmid pET28a::pau20 (sequencing verification) .
[0170] (2) The recombinant plasmid pET28a::pau20 was introduced into Escherichia coli E. coli BL21(DE3) to obtain the recombinant bacterium BL21(DE3)::pET28a-pau20.
[0171] (3) Cultivate the recombinant bacteria BL21(DE3)::pET28a-pau20 in LB liquid medium containi...
Embodiment 3
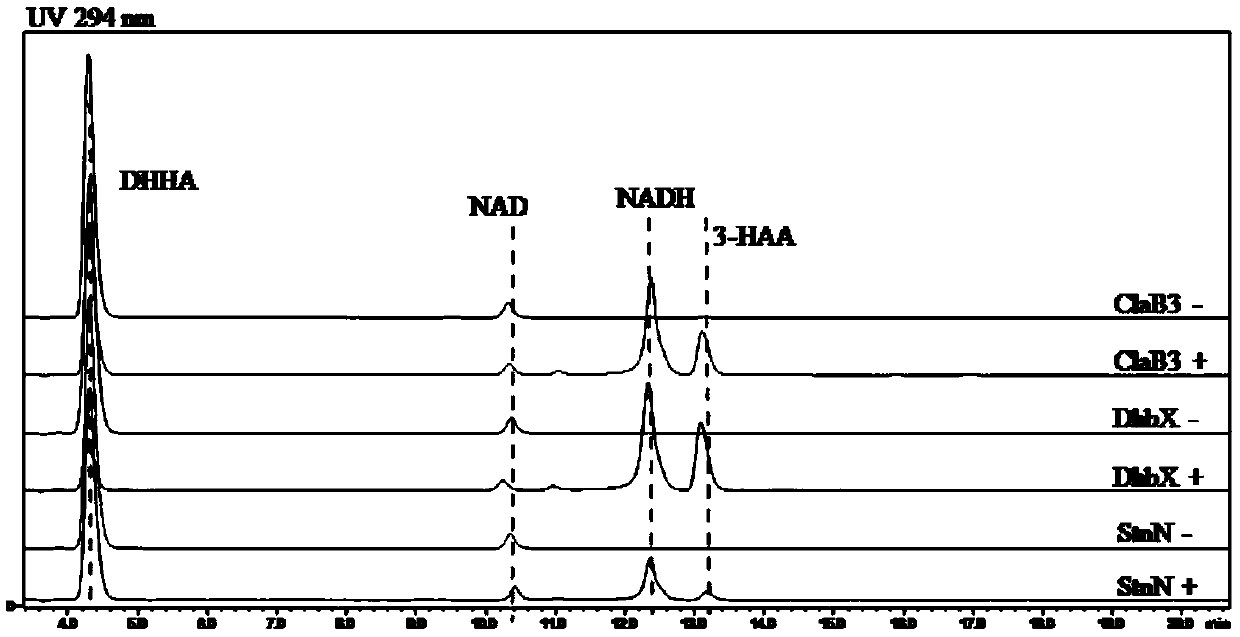
[0202] Embodiment 3, the construction of NAD synthesis pathway plasmid
[0203] The gene sequences of phzD, phzE, nabC and pau20 were optimized according to the codon preference of the heterologous expression strain Escherichia coli, and then connected to the plasmid pXB1a (references: Cui Q, Zhou F, Liu W, et al.Avermectin biosynthesis:stable functional expression of branched chainα-keto acid dehydrogenase complex from Streptomyces avermitilis,in Escherichiacoli,by selectively regulating individual subunit gene expression[J].Biotechnology Letters,2017,39(10):1-8.; the public can get from China obtained from the Institute of Microbiology, Chinese Academy of Sciences) to obtain recombinant plasmids.
[0204] The double-stranded DNA molecule shown in Sequence 3 was used to replace the fragment between NcoI and EcoRI restriction sites of plasmid pXB1a to obtain plasmid pXB1a-HAA (sequenced and verified).
[0205] In sequence 3 of the sequence listing, the 1st to 786th from the 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com