Polyamide-imide drug-loaded nanoparticle and application thereof
A drug-loaded nano-imide technology, applied in the field of anti-tumor drugs, can solve the problems of unguaranteed therapeutic effect, cumbersome multi-step synthesis and availability of purification, and achieve the effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
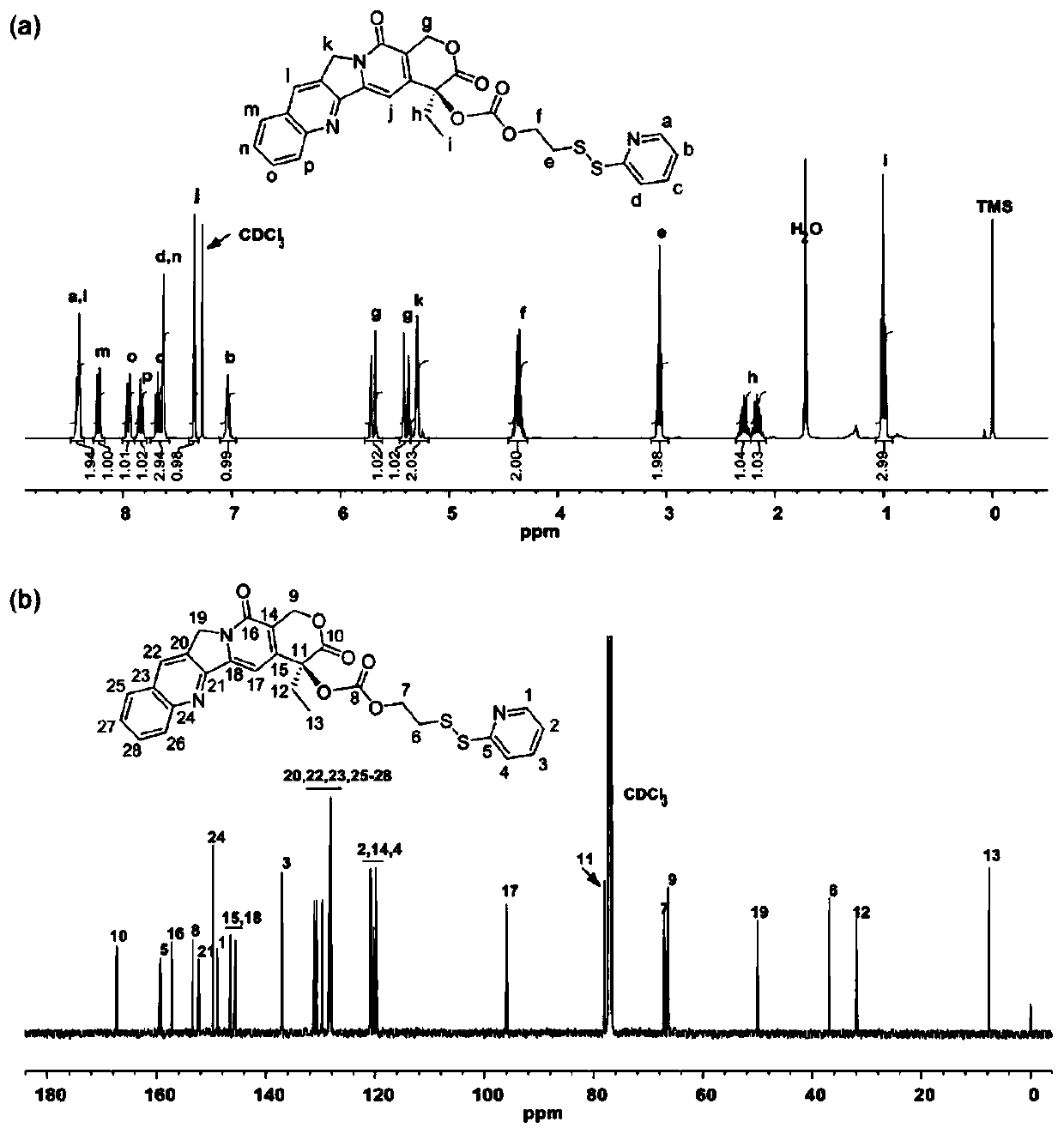
[0044] Synthesis of DSSP-OH: Dissolve 2,2'-dipyridyl disulfide (5.6 g, 25.5 mmol) in 30 mL of methanol, add 0.5 mL of glacial acetic acid as a catalyst, add mercaptoethanol (1.34 g, 17.1 mmol) in 10 ml of methanol, and stirred at room temperature in the dark for 3 hours, after the reaction, the mixture was evaporated in vacuo to a yellow oil, using ethyl acetate: hexane = 3: 7 (v / v) The eluent, the crude product was purified by silica gel chromatography, yield: 54.8%, 1 HNMR (400MHz, chloroform-d) δ8.52(ddd, 1H), 7.61(m, 1H), 7.40(dt, 1H), 7.15(m, 1H), 5.77(t, 1H), 3.83(m, 2H ), 2.95(m, 2H);
[0045] Synthesis of CPT-DSSP: CPT (2.0 g, 5.74 mmol), 4-dimethylaminopyridine (DMAP, 2.10 g, 17.2 mmol) and triphosgene (0.567 g, 1.92 mmol) were suspended in 50 mL of anhydrous dichloromethane , and stirred at room temperature under argon for 30 minutes, then, a solution of DSSP-OH (1.18 g, 6.31 mmol) dissolved in anhydrous tetrahydrofuran (THF) was added dropwise to the above mixture...
Embodiment 2
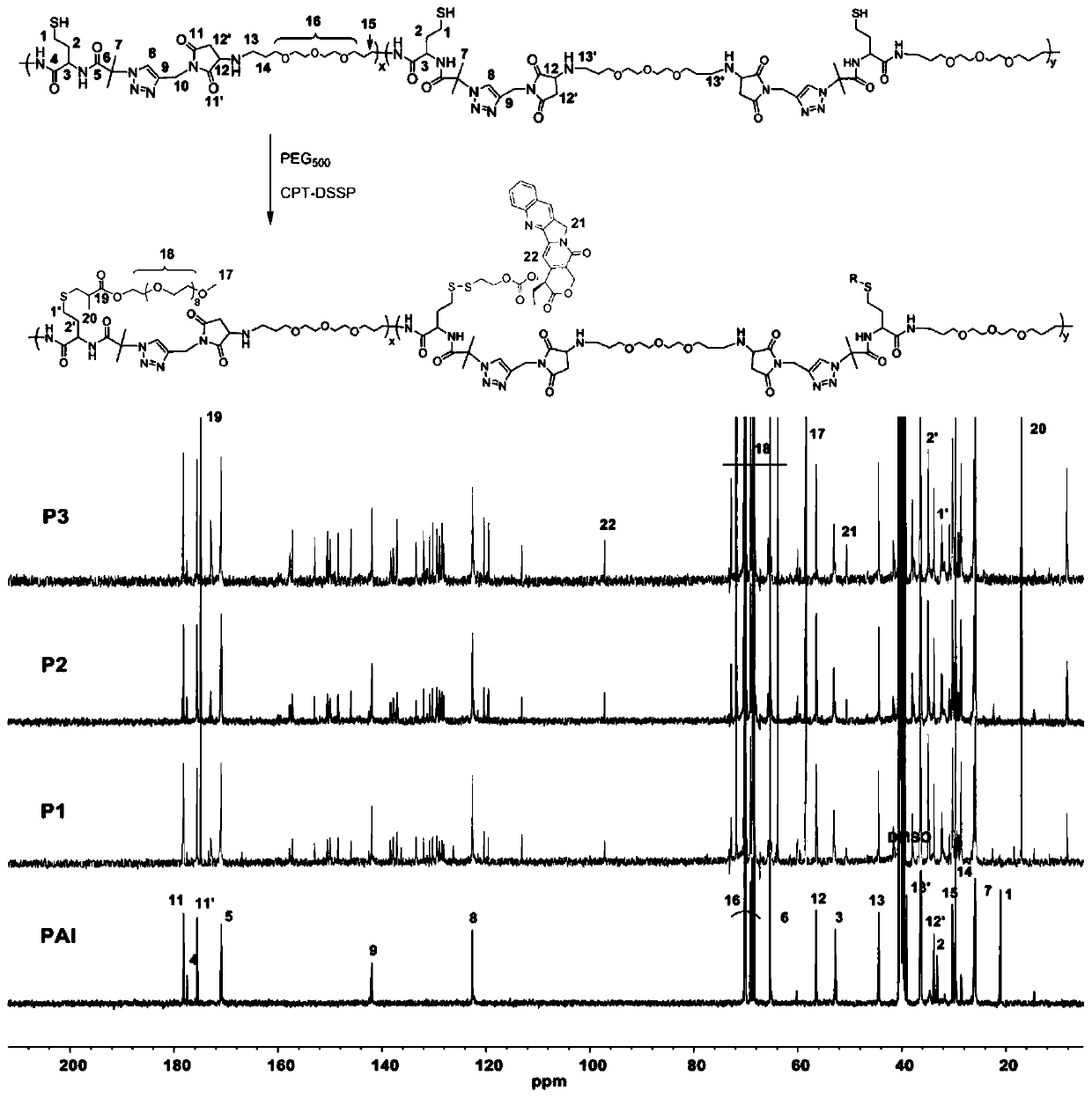
[0053] In vitro drug release: reduction-responsive drug release kinetics from P1-P3 PDNPs by dialysis method. Typically, 1 mL of P1-P3 PDNPs stock solution was added to dialysis tubing (MWCO: 3500 Da) and dialyzed against PBS (10 mM) at 37 °C. For each PDNPs, different concentrations of GSH (2 µM, 5 mM and 10 mM) were used in dialysis. At various predetermined time points, aliquots of the solution were collected and replaced with the same volume of PBS. According to the fluorescence spectrophotometer (E x =370nm,E m = 435nm) to calculate the amount of released CPT. In addition to DOX fluorescence detection wavelength, E x =488nm,E m =591nm, the in vitro drug release of dual-loaded P1-P3 PDNPs was determined by the same method.
[0054] Cell culture: Mouse breast cancer cells (4T1 cells), cervical cancer cells (HeLa cells) and human fibrosarcoma cells (HT-1080 cells) were obtained from the cell bank of Shanghai Institute of Cell Biology, China. Cells were cultured in Dul...
Embodiment 3
[0059] The experimental result of embodiment 1 and embodiment 2:
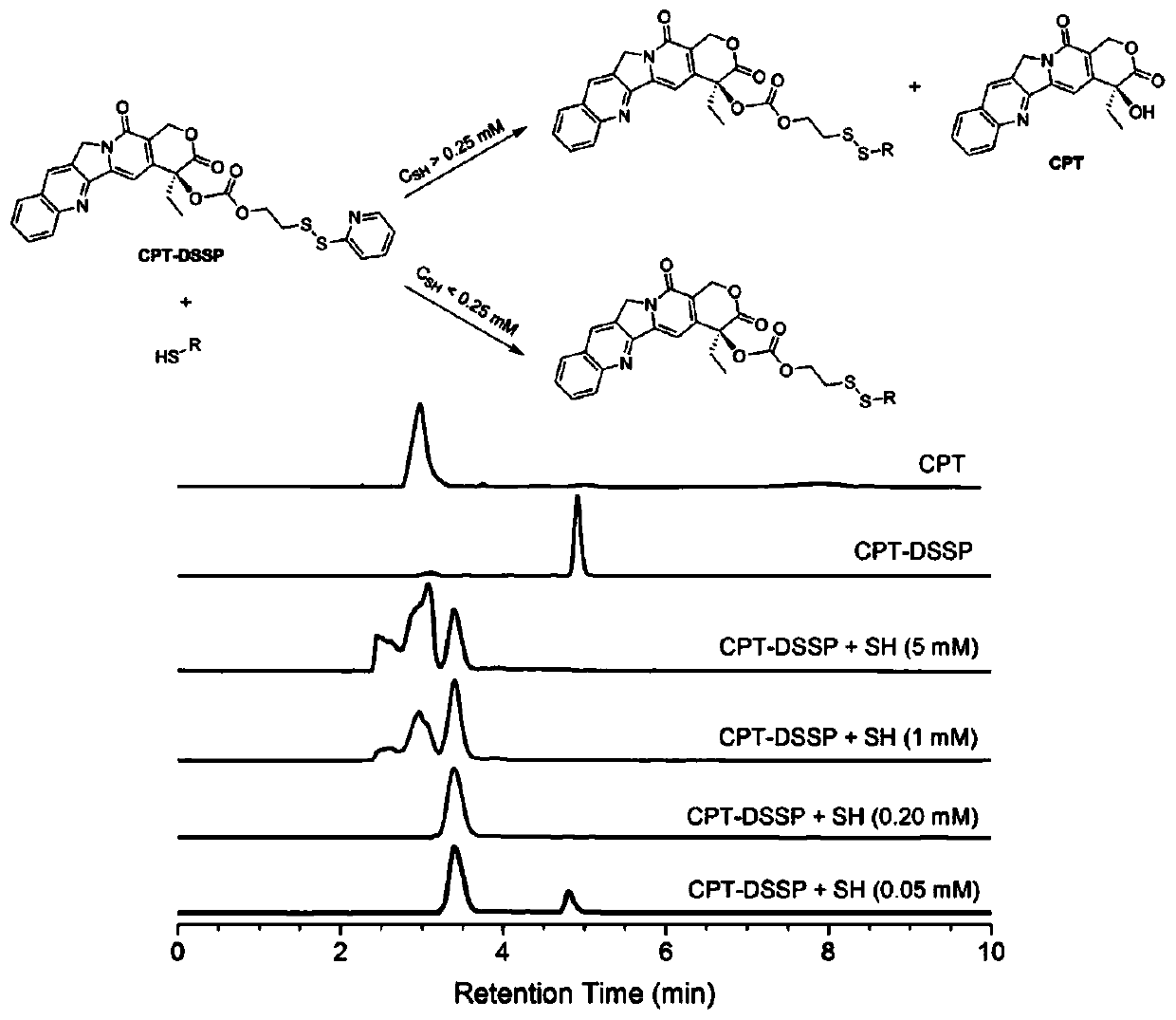
[0060]Synthesis and characterization of PAI-CPT prodrug: This study used the previous research method to combine thiolactone maleimide monomer with 4,7,10-trioxy-1,13-tridecanediamine (diamine 220) Equimolar ratio condensation polymerization to synthesize aliphatic PAI (polyamide-imide), the synthetic route diagram is shown in figure 2 . Quantitative characterization of the aminolysis of thiolactone and amine-maleimide-Michael additions allows a very efficient synthesis of PAIs and facilitates the in situ one-pot modification of PAIs. Prior to PAI-CPT coupling, pyridyl disulfide-modified CPT (i.e. CPT-DSSP) prodrugs were synthesized by a two-step process with an overall yield of about 33%, 1 H and 13 C NMR results confirmed its chemical structure (see figure 1 ).
[0061] In the present invention, due to the presence of the pyridine disulfide moiety, the CPT-DSSP prodrug can not only release CPT in a redu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Surface potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com