A class of naphthoquinone pyranoindole derivatives, preparation method and applications thereof
A technology of indole derivatives and naphthoquinones, applied in chemical instruments and methods, drug combinations, instruments, etc., to achieve the effects of simple synthesis, excellent fluorescence imaging performance, and good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
[0029] Weigh 1 mmol of 2-hydroxy-1,4-naphthoquinone, 1 mmol of substituted o-bromobenzaldehyde, 1 mmol of isonitrile, 0.2 mmol of L-proline, 0.2 mmol of cuprous iodide, and 2 mmol of potassium carbonate into a 100 mL round bottom flask In the middle, after vacuum treatment and flushing with argon, add 30 mL of dry treated toluene, heat and stir under reflux for 36 hours, cool to room temperature after the reaction is completed (TLC tracking), and spin dry the excess solvent to obtain the crude product, which is separated and purified by column chromatography ( Petroleum ether: ethyl acetate = 20:1), the target compound 5 was obtained.
[0030] Take compound 5a as an example:
[0031] Weigh 1 mmol of 2-hydroxy-1,4-naphthoquinone, 1 mmol of o-bromobenzaldehyde, 1 mmol of cyclohexylisonitrile, 0.2 mmol of L-proline, 0.2 mmol of cuprous iodide, and 2 mmol of potassium carbonate into a 100 mL round bottom In the flask, after vacuum treatment and flushing with argon, ad...
Embodiment 2
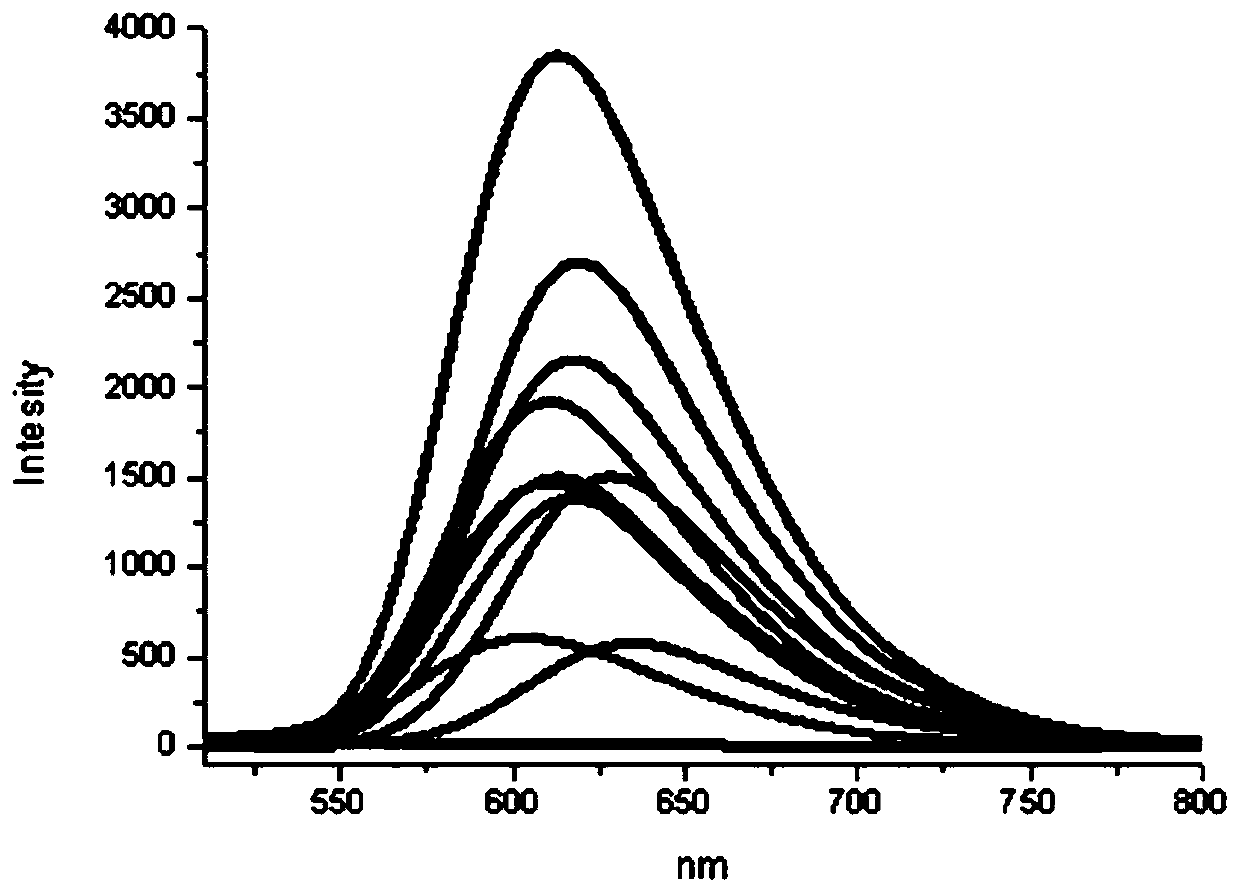
[0126] Using ethyl acetate as solvent, the compound 5 was formulated into 1×10 -5 mol L -1 The dilute solution of the test UV absorption spectrum is shown in Figure 1. Depend on figure 1It can be seen that the ultraviolet absorption wavelength of compound 5 is in the range of 495-525nm, entering the visible light region, indicating that there is a relatively long conjugated system in the molecule of 5, and the charge transfer is very smooth and easy. The absorption peaks appear in the visible light region, indicating that the series of compounds have less damage to living cells, and have the potential to be further prepared into biological probes and imaging reagents.
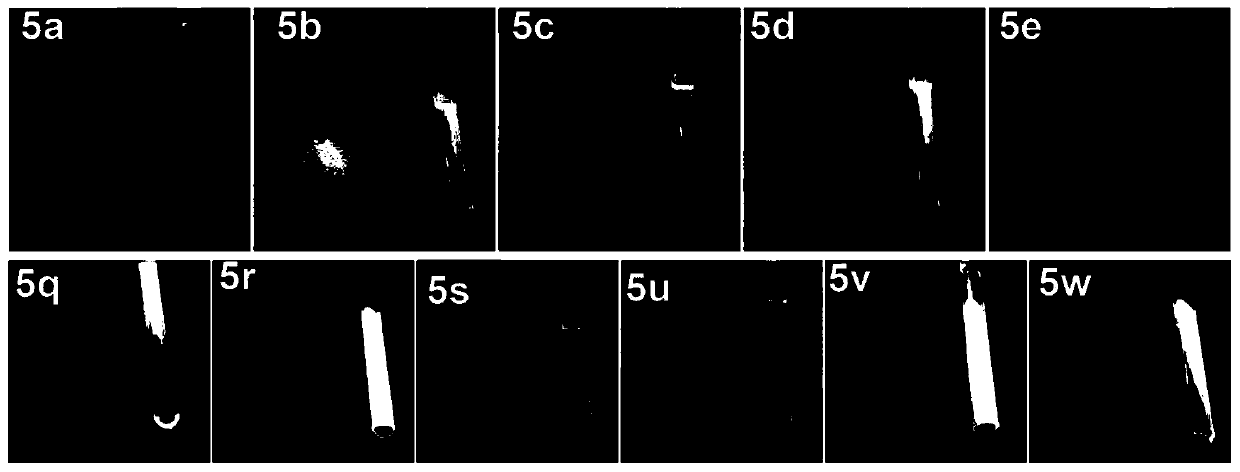
[0127] The photos of the solution and solid state of compound 5 under the irradiation of 365nm ultraviolet lamp are as follows: figure 2 As shown, in each photo, the left side is the solid of the compound, and the right side is the solution of the compound, obtained by dissolving in chloroform as a solvent....
Embodiment 3
[0131] The antitumor activity of embodiment 3 products
[0132] MTT method was used to preliminarily test the effect of compound 5 on human triple-negative breast cancer cells (MDA-MB-231), human liver cancer cells (HepG-2), human lung cancer cells (A549), human liver immortalized cells (THLE) and human Cytotoxicity of bronchial epithelial cells (HBE), test results show that only 5b has inhibitory effect on MDA-MB-231 and A549, and is also toxic to normal cells. If it is to be further developed into an anti-tumor drug, the structure must be modified .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com