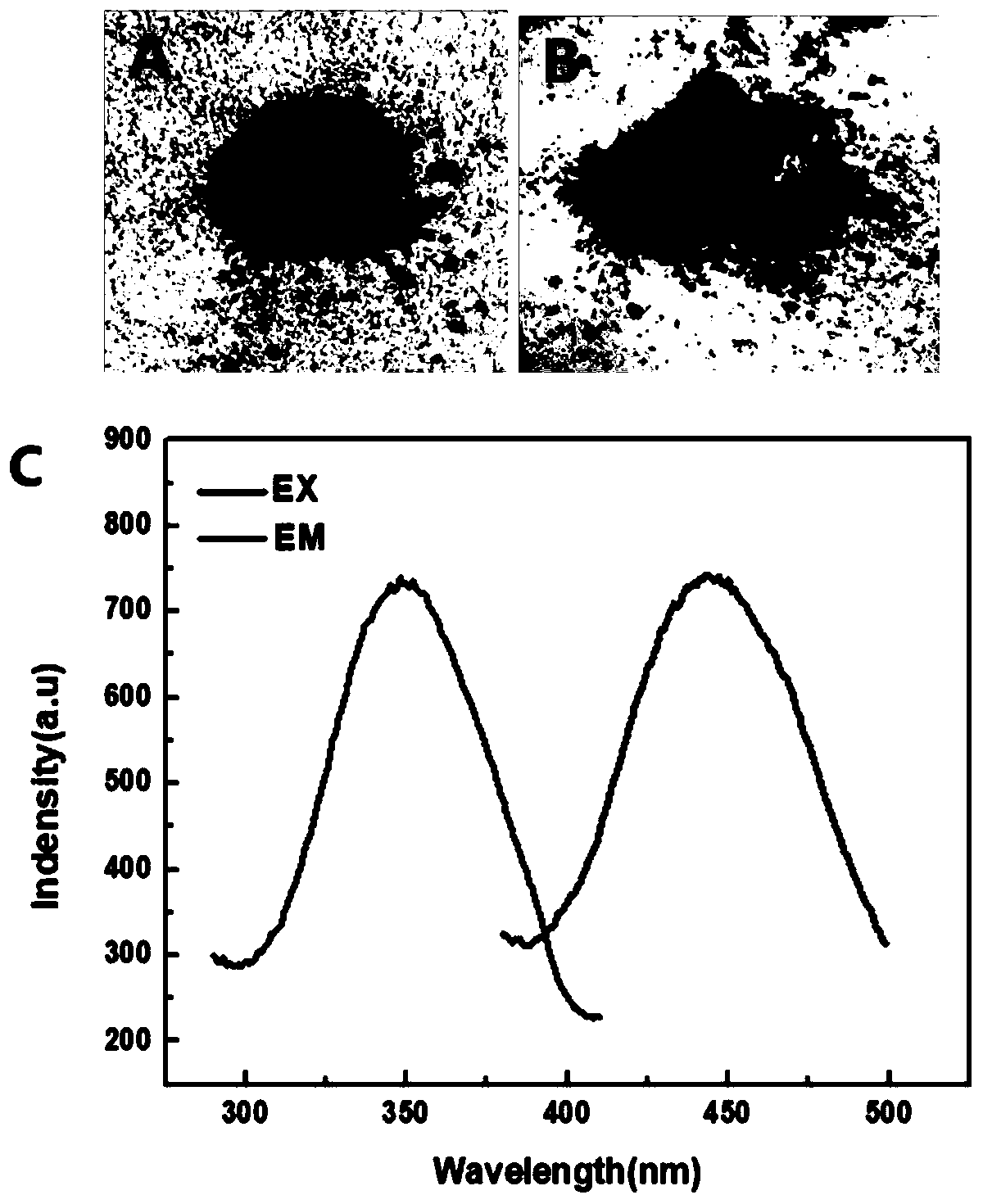
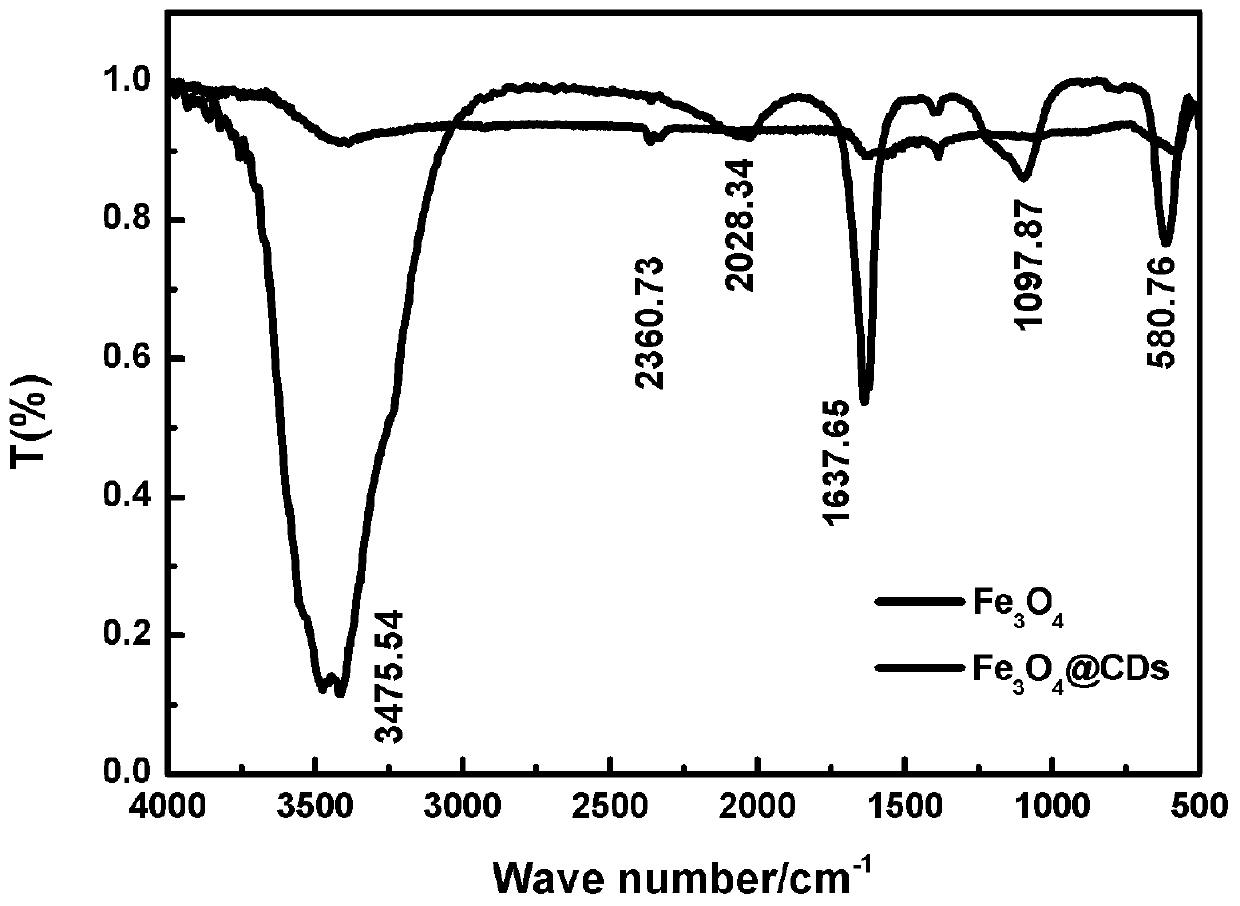
Preparation method of difunctional magnetic fluorescent nanocomposite Fe3O4@CDs microspheres
A fluorescent nano and dual-functional technology, applied in the direction of magnetic materials, magnetic objects, nanotechnology, etc., can solve the problems of low chemical stability, potential toxicity, and narrow suitable range of quantum dots, and achieve less restrictions on reaction conditions and stability Good performance and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1. Fe 3 o 4 Preparation of:
[0025] Weigh 0.2g of ferric chloride hexahydrate (FeCl 3 ﹒ 6H 2 O), add 15mL deionized water to dissolve, then add 0.5g citric acid monohydrate, 3mL ethylenediamine, 0.3g sodium hydroxide. After stirring evenly, transfer to a 50mL reactor and react at 200°C for 12h. The black solution was magnetically separated and washed several times with ethanol and deionized water to remove residual reactants, and dried in vacuum at 60°C for 6 hours.
[0026] 2. Fe 3 o 4 @SiO 2 -NH 2 Preparation of:
[0027] 0.1g Fe 3 o 4 Disperse in 100mL ethanol solution (ethanol / water = 1 / 4, V; V), add 1.5mL ammonia water, ultrasonic 15min, make it evenly dispersed in the solution, then slowly add 1mL tetraethyl orthosilicate under mechanical stirring, After reacting for 45min, 0.07mL of 2-(aminoethyl)propyltrimethylsilane was added dropwise, stirring was continued for 4h at room temperature, and the synthesized Fe 3 o 4 @SiO 2 -NH 2 After the nanopa...
Embodiment 2
[0032] 1. Fe 3 o 4 Preparation of:
[0033] Weigh 0.3g of ferric chloride hexahydrate (FeCl 3 ﹒ 6H 2 O), add 10mL deionized water to dissolve, then add 0.6g citric acid monohydrate, 2mL ethylenediamine, 0.4g sodium hydroxide. After stirring evenly, transfer to a 50mL reactor and react at 200°C for 8h. The black solution was magnetically separated and washed several times with ethanol and deionized water to remove residual reactants, and dried in vacuum at 60°C for 6 hours.
[0034] 2. Fe 3 o 4 @SiO 2 -NH 2 Preparation of:
[0035] 0.1gFe 3 o 4 Disperse in 100mL ethanol solution (ethanol / water=10 / 3, V; V), add 1.5mL ammonia water, ultrasonic 15-30min to make it evenly dispersed in the solution, then slowly add 1mL ethyl orthosilicate under mechanical stirring After reacting for 45-60min, add 0.07mL of 2-(aminoethyl)propyltrimethylsilane dropwise, and continue stirring at room temperature for 4-6h, and the synthesized Fe 3 o 4 @SiO 2 -NH 2 After the nanoparticle...
Embodiment 3
[0040] 1. Fe 3 o 4 Preparation of:
[0041] Weigh 0.2g of ferric chloride hexahydrate (FeCl 3 ﹒ 6H 2 O), add 15mL deionized water to dissolve, then add 0.5g citric acid monohydrate, 3mL ethylenediamine, 0.3g sodium hydroxide. After stirring evenly, transfer to a 50mL reactor and react at 200°C for 12h. The black solution was magnetically separated and washed several times with ethanol and deionized water to remove residual reactants, and dried in vacuum at 60°C for 6 hours.
[0042] 2. Fe 3 o 4 @SiO 2 -NH 2 Preparation of:
[0043] 0.16g Fe 3 o 4 Disperse in 130mL ethanol solution (ethanol / water=10 / 3, V; V), add 2mL ammonia water, ultrasonic 30min, make it evenly dispersed in the solution, then slowly add 2mL tetraethyl orthosilicate under mechanical stirring, react After 60min, 0.66mL of 3-aminopropyltriethoxysilane was added dropwise, stirring was continued for 6h at room temperature, and the synthesized Fe 3 o 4 @SiO 2 -NH 2 After the nanoparticles were sep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com