Catalyst for preparing sulfur by selective oxidation of hydrogen sulfide and preparation method thereof
A selective oxidation and catalyst technology, applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc., can solve the problem of poor resistance to sulfation and oxygen fluctuation, The problems of low catalyst selectivity and low sulfur recovery rate can achieve the effect of strong anti-sulfation and anti-oxygen content fluctuation ability, good economic and environmental benefits, and long catalyst life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Weigh 160g of titanyl sulfate and add a certain amount of water to make it completely dissolved, add 67g of silica sol containing 30% (wt), stir to obtain a mixed solution, add dropwise 25% (wt) ammonia solution, and control the terminal pH to be 10-11. The resulting suspension was left to stand for 4 hours, filtered and washed, and the obtained solid was dried at 100°C, calcined at 500°C for 4 hours, and pulverized to obtain a titanium-silicon composite oxide, coded as TS-1.
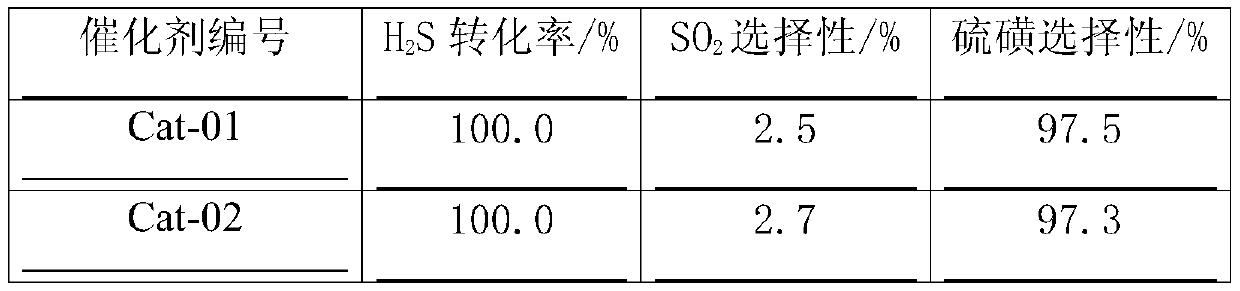
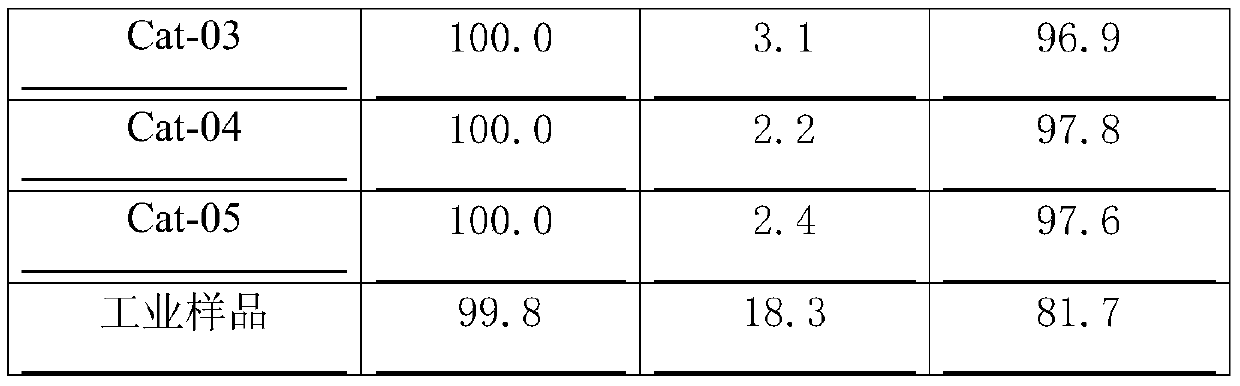
[0030] Weigh 89g titanium-silicon composite oxide TS-1, add 25g Fe(NO 3 ) 3 9H 2 O and 3.7g Al(NO 3 ) 3 9H 2 The aqueous solution made of O was stirred and impregnated. After the mud body was aged for 12 hours, it was dried at 100°C. After crushing, 50g and 2g of scallop powder were extruded into strips. After decomposing at 500°C for 4 hours, the finished catalyst was obtained, and the number was Cat-01. .
Embodiment 2
[0032] Weigh 89g titanium-silicon composite oxide TS-1, add 25g Fe(NO 3 ) 3 9H 2 The solution prepared by O was stirred and impregnated. After the mud body was aged for 12 hours, it was dried at 100°C, crushed, added with 4g of scallop powder and extruded into strips, decomposed at 500°C for 4 hours to obtain a semi-finished catalyst. Get 99g of the above semi-finished products and add to 3.7g Al(NO 3 ) 3 9H2 Immerse equal volumes in the aqueous solution made of O, dry at 60°C, and decompose at 400°C for 2 hours to obtain the finished catalyst, numbered Cat-02.
Embodiment 3
[0034] Weigh 89g of titanium-silicon composite oxide TS-1, mix it with 4g of Sesame powder, add 6.4g of Mg(NO 3 ) 2 ·6H 2 The aqueous solution made of O was extruded, aged at room temperature for 12 hours, dried at 45°C, and decomposed at 500°C for 4 hours to obtain a catalyst carrier. Get above-mentioned carrier 90g and add to with 25g Fe(NO 3 ) 3 9H 2 The solution prepared by O was impregnated with equal volume, dried at 60°C, and decomposed at 400°C for 2 hours to obtain the finished catalyst, numbered Cat-03.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com