Preparation method of fluorine-doped porous carbon nanofiber loaded alkali metal hydrogen storage material
A technology of nanofibers and hydrogen storage materials, applied in the field of hydrogen storage, can solve the problems of reduced hydrogen storage capacity, easy agglomeration of alkali metals, etc., and achieve the effects of improving hydrogen storage performance and reducing dehydrogenation temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The invention provides a method for preparing a fluorine-doped porous carbon nanofiber loaded alkali metal hydrogen storage material, comprising the following steps:
[0026] (1) Immerse polyacrylonitrile (PAN), polyvinylpyrrolidone (PVP) and perfluorosulfonic acid (PFSA) powders in N,N-dimethylformamide (DMF), and stir the mixed system at room temperature for 20 ~48 hours; wherein the mass ratio of polypropylene polyacrylonitrile to polyvinylpyrrolidone is 1:1 or 2:1; PFSA powder accounts for 1~5wt% of the total weight of the PAN and PVP. The PAN, PVP and PFSA powders in the mixed solution account for 8-12wt% of the total weight of the solution.
[0027] (2) Dispersing the mixed solution uniformly in step (1) to prepare porous nanofibers by electrospinning or hydrothermal method;
[0028] (3) replacing the porous PAN / PFSA nanofibers in step (2) with an alkali metal solution to form lithium / calcium-porous PAN / PFSA nanofibers;
Embodiment 1
[0031] 1. Prepare spinning solution, take 0.5gPAN, 0.5gPVP (1:1) and 0.01g PFSA into 9gDMF for mixing and dissolving, the mass fraction of the total weight of PAN, PVP and PFSA powder in the mixed solution is 10wt% ; at 25 o C, stirred for more than 24h, completely dissolved to form a uniform spinning solution.
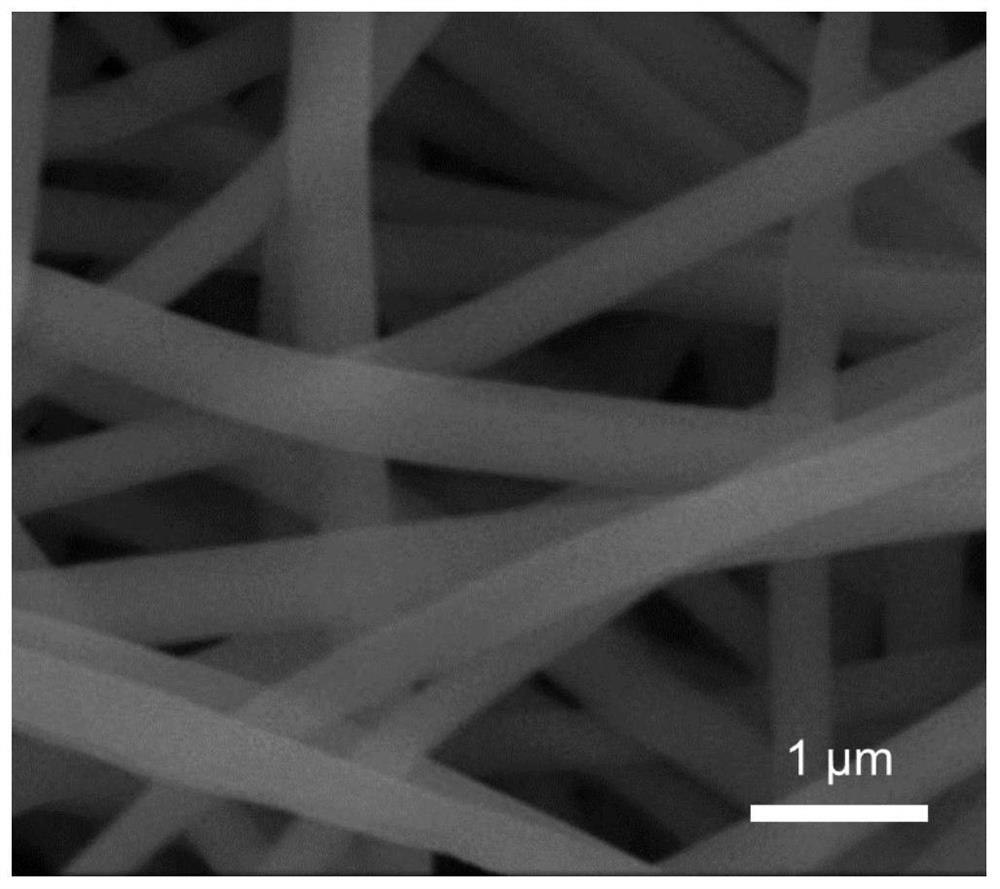
[0032] 2. Preparation of composite fibers by electrospinning: at a voltage of 18 kV, a receiving distance of 15 cm, a spinning solution delivery rate of 0.2 mL / h, and an electrospinning temperature of 30 o Under the condition of C, PAN / PVP / PFSA composite nanofibers (such as figure 1 shown), placed in a vacuum oven at 80 o C, dry for 8h.
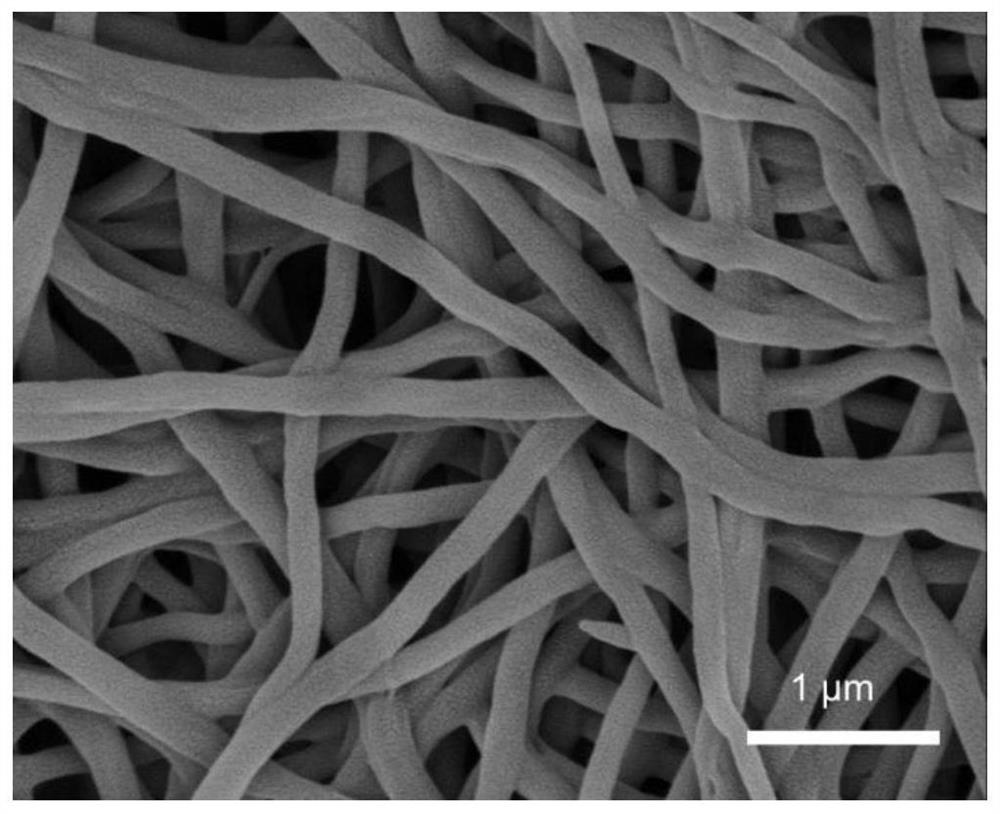
[0033] 3. Porous composite fiber: soak the PAN / PVP / PFSA composite nanofiber in water, put it in an autoclave, then place it in a muffle furnace, 110 o C, after 24h, filter and dry to obtain porous PAN / PFSA nanofibers.
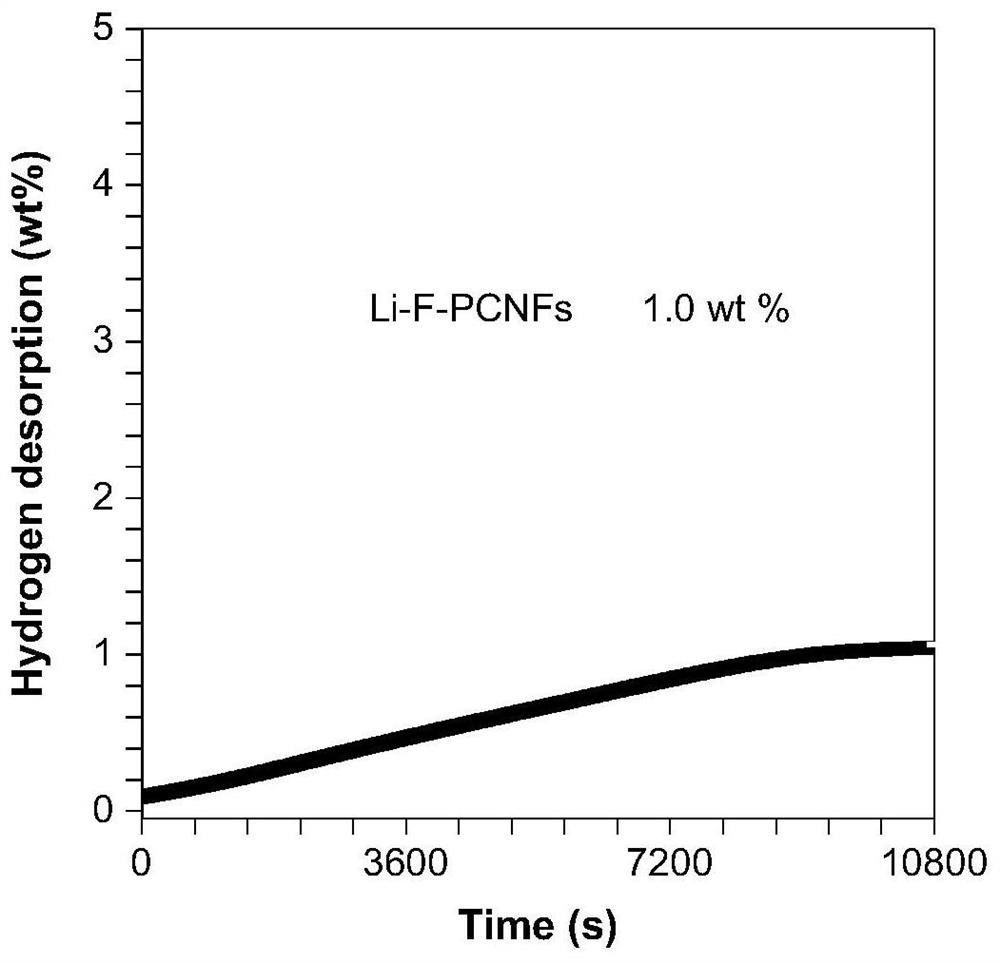
[0034] 4. Li-porous PAN / PFSA nanofibers: soak the porous PAN / PFSA nanofibers in (0.01g / mL) lithium hydroxide s...
Embodiment 2
[0039] 1. Prepare spinning solution, take 1gPAN, 0.5gPVP (2:1) and 0.05g PFSA into 13.5gDMF for mixing and dissolving, the mass fraction of the total weight of PAN, PVP and PFSA powder in the mixed solution is 10wt% ; at 25 o C, stirred for more than 24h, completely dissolved to form a uniform spinning solution.
[0040] 2. Preparation of composite fibers by electrospinning: at a voltage of 18 kV, a receiving distance of 15 cm, a spinning solution delivery rate of 0.2 mL / h, and an electrospinning temperature of 30 o Under the condition of C, obtain PAN / PVP / PFSA composite nanofiber by electrospinning, place 80 in vacuum oven o C drying 8h.
[0041] 3. Porous composite fiber: soak the PAN / PVP / PFSA composite nanofiber in water, put it in an autoclave, then place it in a muffle furnace, 110 o C, after 24h, filter and dry to obtain porous PAN / PFSA nanofibers.
[0042] 4. Li-porous PAN / PFSA nanofibers: soak the porous PAN / PFSA nanofibers in (0.01g / mL) lithium hydroxide solution,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com