Preparation method of recombinant halophilic archaea protease
A technology of halophilic archaea and protease, applied in the field of bioengineering, can solve the problems of low expression of extracellular enzymes, denaturation and inactivation, slow growth of halophilic archaea, etc., and achieves simple purification steps, short production cycle, good general-purpose sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
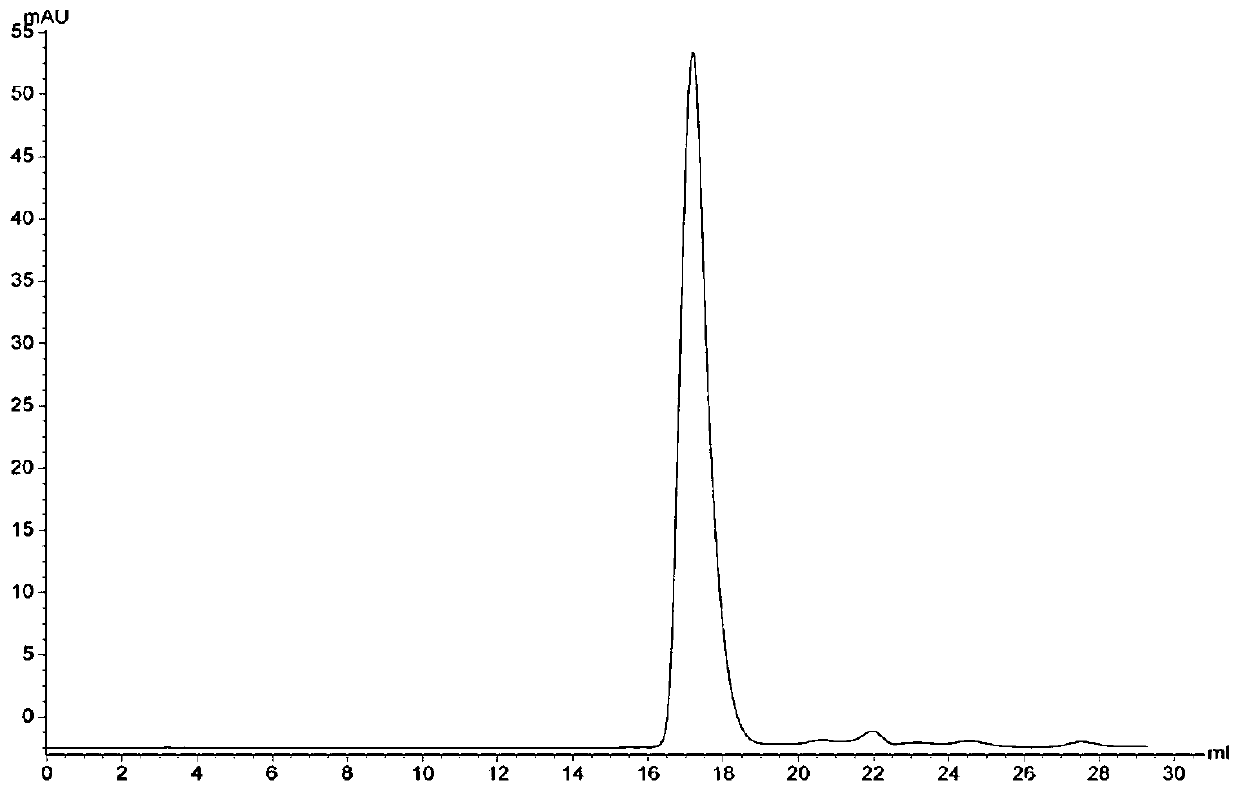
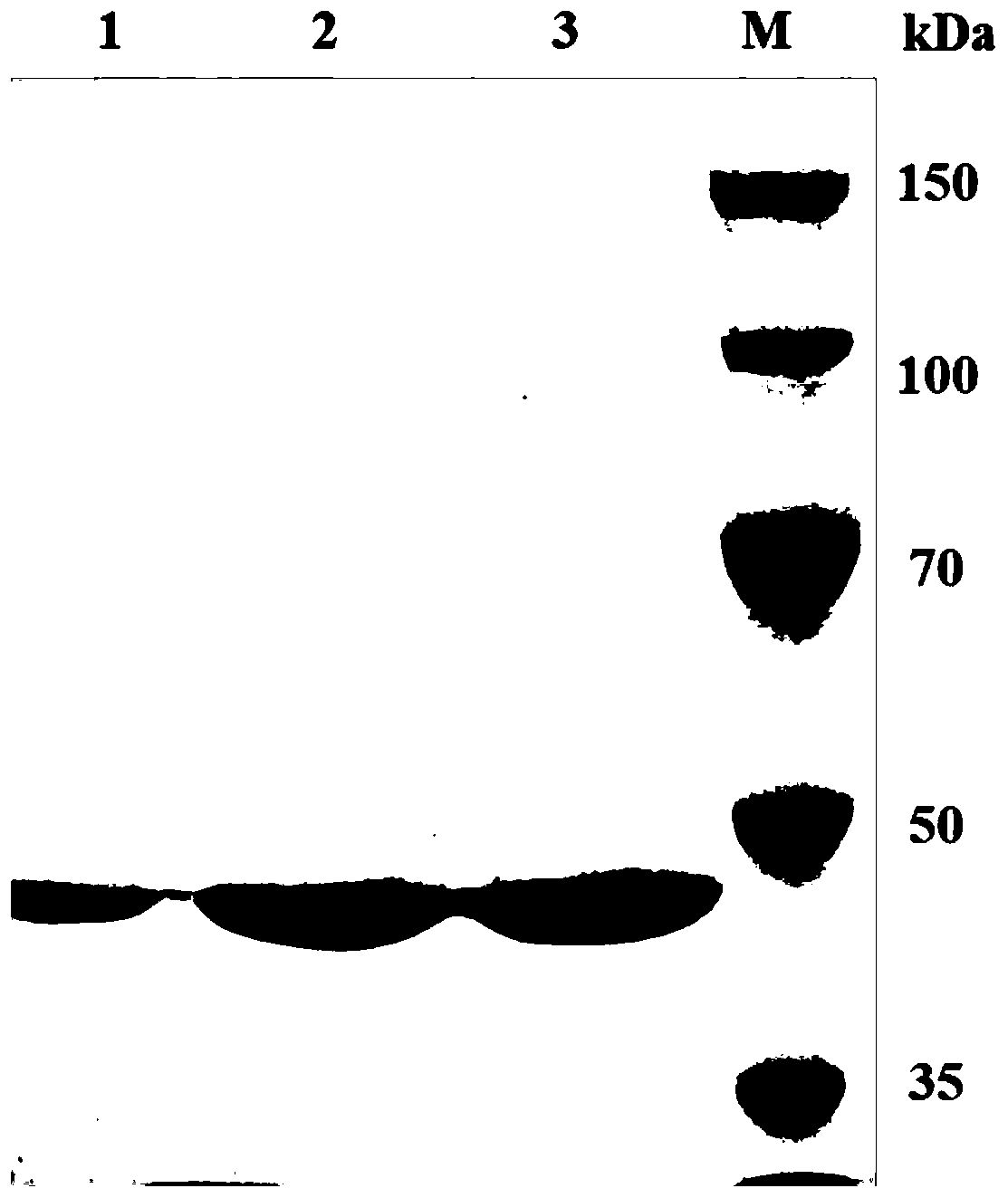
Image
Examples
Embodiment 1
[0036] Heterologous expression of recombinant halophilic archaeal protease (HPDC1);
[0037] (1) Target gene PCR amplification
[0038] Use a sterile inoculation loop to take a ring of strain Halostella sp.DL-M4 cells (isolated from salted kelp, preserved in CGMCC 1.13603) in 30 μL of sterile water, boil for 10 minutes, centrifuge briefly and take the supernatant as a PCR template; specificity Primer forward primer F sequence is 5'-ATA CCATGG CAAATAATGGCAACCCAT-3', reverse primer R sequence is 5'-ATA CTCGAG GCCACCGCCGTTGCCGAC-3', the underlined sequences are NcoI restriction site and XhoI restriction site, respectively. The enzyme used for PCR amplification was KOD-PLUS (purchased from TOYOBO Company), and the amplification system was 50 μL.
[0039]The dosage of each ingredient is as follows:
[0040]
[0041]
[0042] PCR amplification program:
[0043]
[0044] (2) Electrophoresis verification gel cutting recovery;
[0045] Use 1% agarose gel electrophoresi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com