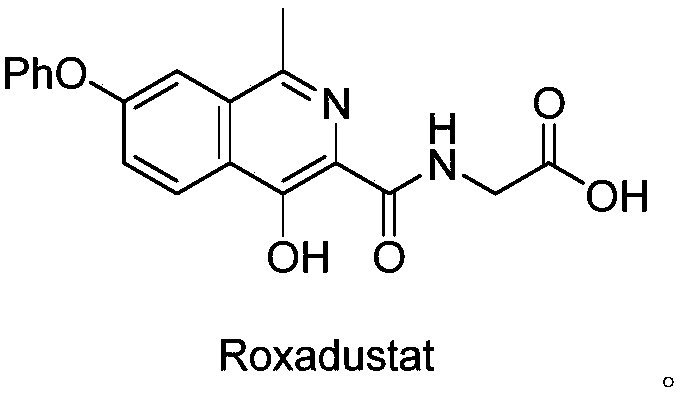
Preparation method of Roxadustat intermediate
A ligand-to-volume ratio technology, applied in the field of preparation of roxadustat intermediates, can solve the problems of cumbersome preparation process, low yield, and high production safety risks, and achieves high reaction yield, easily available raw materials, and a process route. Mature and controllable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
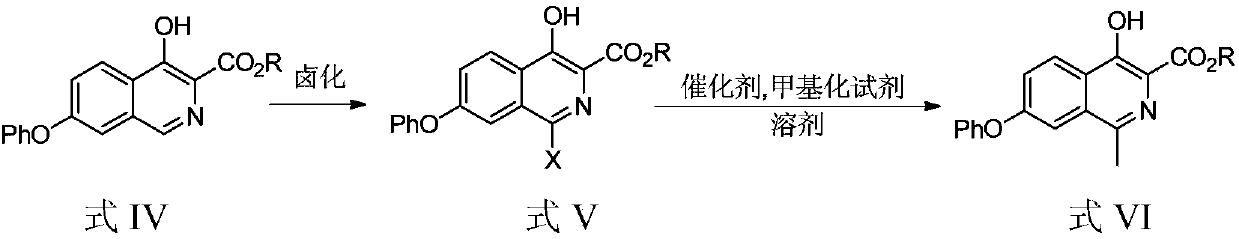
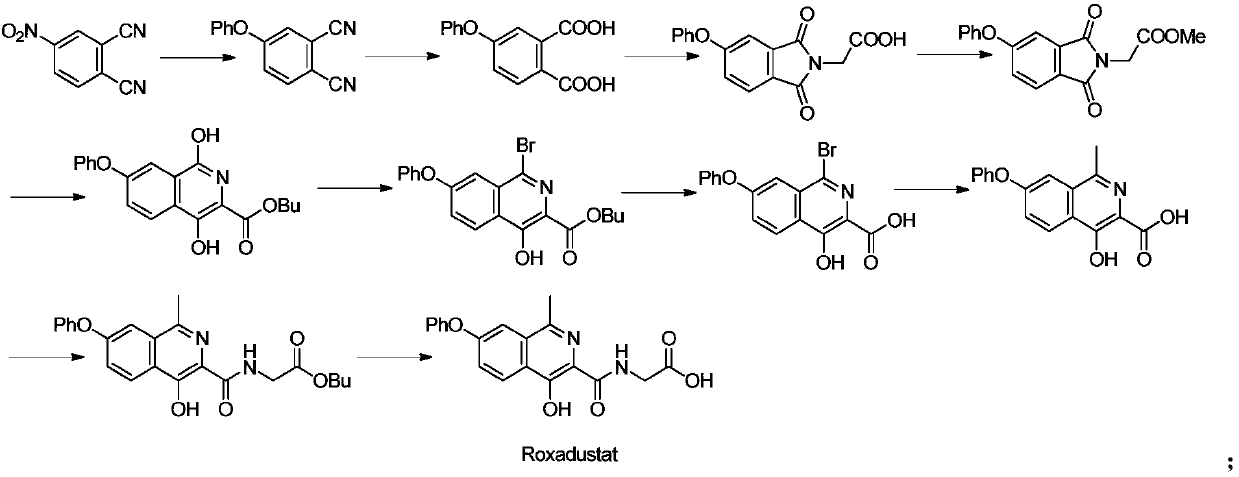
[0065] Experimental example 1: Preparation of methyl 1-bromo-4-hydroxy-7-phenoxyisoquinoline-3-carboxylate
[0066]
[0067] Method 1: Add dichloromethane (2L) and methyl 4-hydroxy-7-phenoxyisoquinoline-3-carboxylate (200.00g, 0.68mol) in turn to the reaction flask, stir well, then add N-bromosuccinimide (NBS) (126.60g, 0.71mol) was added into the reaction flask. After the addition, it was reacted for 2 hours. After about half of the dichloromethane was removed by distillation under reduced pressure, methanol (2L) was added and analyzed. The crystal was stirred for 1 h, filtered, and the filter cake was air-dried to obtain methyl 1-bromo-4-hydroxy-7-phenoxyisoquinoline-3-carboxylate (229.67 g, yield 90.7%, purity 98.98%).
[0068] Method 2: Add acetonitrile (200mL) and 4-hydroxy-7-phenoxyisoquinoline-3-methyl carboxylate (20.00g, 67.72mmol) in turn to the reaction flask, stir well, and then add N- Bromosuccinimide (NBS) (12.66g, 71.11mmol) was added to the reaction flask. ...
experiment example 2
[0070] Experimental example 2: Preparation of methyl 1-methyl-4-hydroxy-7-phenoxyisoquinoline-3-carboxylate
[0071]
[0072] Method 1: Add toluene (5.2L) into the reaction flask, start stirring, deoxidize under nitrogen protection for 30min, then add 1-bromo-4-hydroxy-7-phenoxyisoquinoline-3-formic acid in sequence under nitrogen protection Ester (200.0g, 0.53mol), palladium acetate (12.0g, 0.053mol), tricyclohexylphosphine (29.9g, 0.10mol), potassium phosphate heptahydrate (904.0g, 2.65mol) and trimethylboroxine Alkane (300ml, 1.06mol), after the addition, react at 90-110°C for 8h. After the reaction, cool down to 0-10°C, add 5.2L of isopropyl acetate, add 3.3L of 1mol / L hydrochloric acid, adjust the pH to 6.0-8.0, and carry out the extraction operation. The aqueous phase is extracted once with dichloromethane (5.2L) , the organic phases were combined, concentrated under reduced pressure, and purified by column (eluent: n-heptane / dichloromethane / ethyl acetate volume rati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com