Green synthesis method for p-iodotoluene
A green synthesis technology of p-iodotoluene, applied in chemical instruments and methods, preparation of halogenated hydrocarbons, disproportionation separation/purification of halogenated hydrocarbons, etc., can solve problems such as high price, hidden dangers of production safety, unfavorable large-scale industrial production, etc. Achieve high yield and reduce production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
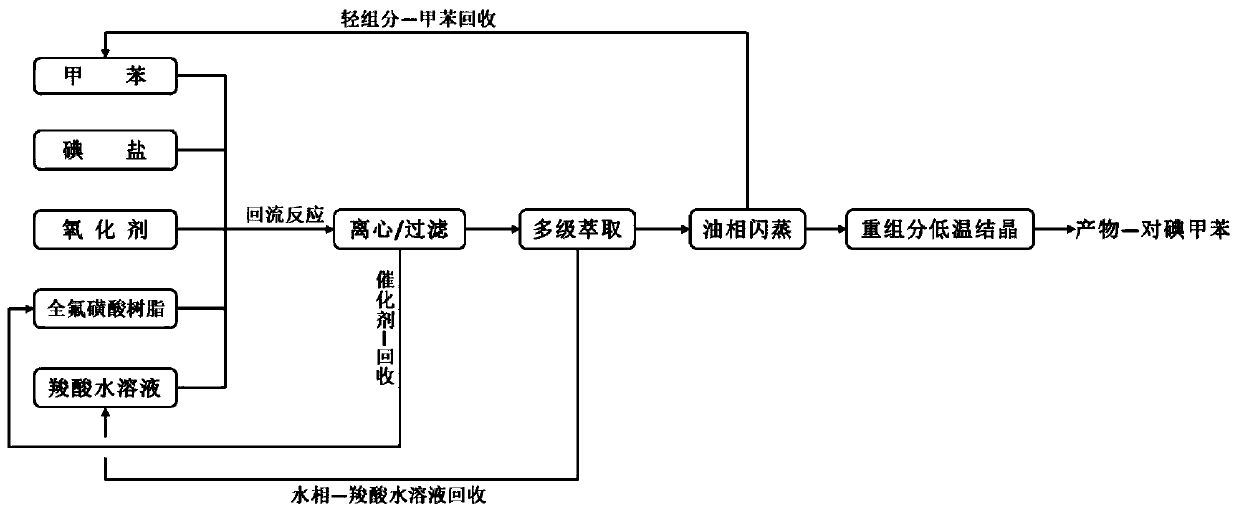
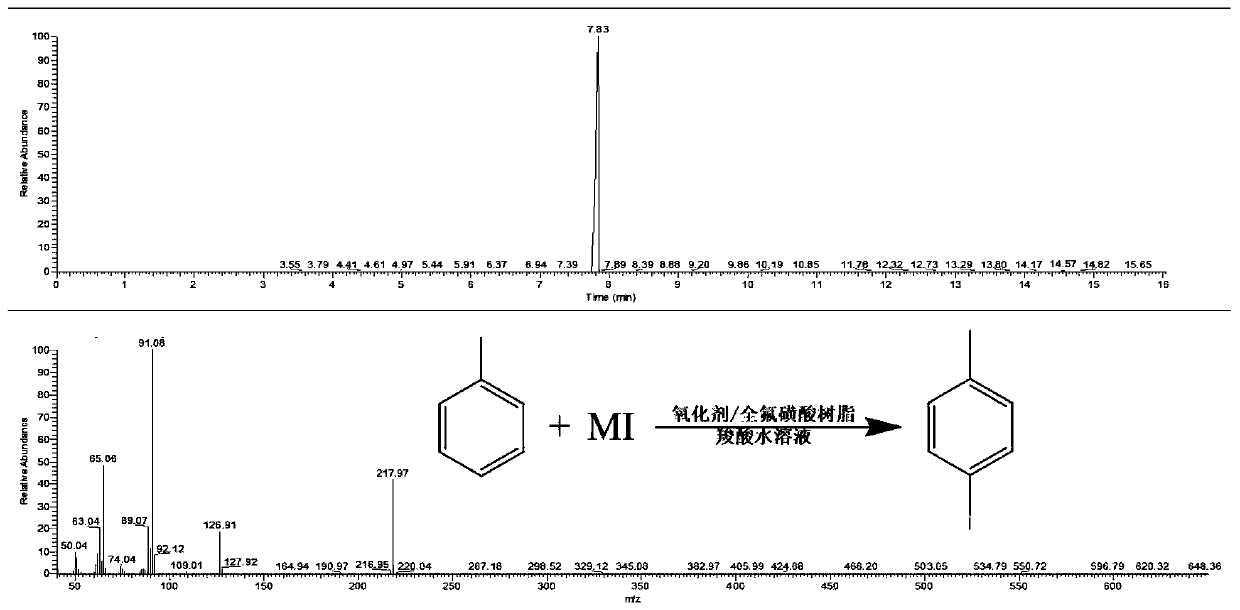
[0032] To a 500mL four-neck flask equipped with a thermometer and a reflux condenser, add 100mL of formic acid aqueous solution with a volume concentration of 20%, 0.2mol (18.4g) of toluene, 0.2mol of hydrogen peroxide, 0.2mol (30g) of sodium iodide and 1.84g of perfluorinated For sulfonic acid resin, turn on the heating, adjust the reaction temperature so that the reaction solution is in a reflux state until the color of the reaction solution changes from purple to colorless, stop the reaction, and recover the perfluorosulfonic acid resin through centrifugation or filtration of the reacted mixed solution, centrifuge Or after filtration, the filtrate is subjected to a multi-stage extraction-flash evaporation-low temperature crystallization process to sequentially separate formic acid aqueous solution, unreacted toluene and product p-iodotoluene, and the yield of p-iodotoluene is 81%.
Embodiment 2
[0034] To a 500mL four-necked flask equipped with a thermometer and a reflux condenser, add 100mL of 30% acetic acid aqueous solution, 0.2mol (18.4g) toluene, 0.1mol potassium persulfate compound salt, 0.1mol (15g) sodium iodide in sequence and 18.4g perfluorosulfonic acid resin, turn on the heating, adjust the reaction temperature so that the reaction solution is in a reflux state, until the color of the reaction solution changes from purple to colorless, stop the reaction, and the reacted mixed solution is recovered by centrifugation or filtration. Sulfonic acid resin, after centrifugation or filtration, the filtrate is subjected to multi-stage extraction-flash evaporation-low temperature crystallization process, and the acetic acid aqueous solution, unreacted toluene and product p-iodotoluene are separated in sequence. After testing, the yield of p-iodotoluene is 86%.
Embodiment 3
[0036] To a 500mL four-neck flask equipped with a thermometer and a reflux condenser, add 100mL of 30% propionic acid aqueous solution, 0.2mol (18.4g) toluene, 0.15mol ammonium persulfate, 0.1mol (16.6g) potassium iodide and 184g all Fluorosulfonic acid resin, turn on the heating, adjust the reaction temperature to keep the reaction solution in a reflux state, until the color of the reaction solution changes from purple to colorless, stop the reaction, and recover the perfluorosulfonic acid resin by centrifuging or filtering the reacted mixed solution. After centrifugation or filtration, the filtrate is subjected to multi-stage extraction-flash evaporation-low temperature crystallization process, and the propionic acid aqueous solution, unreacted toluene and product p-iodotoluene are sequentially separated. After testing, the yield of p-iodotoluene is 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com